286281
Indole-3-acetamide
98%
Synonym(s):
3-Indolylacetamide, NSC 1969
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10N2O
CAS Number:
Molecular Weight:
174.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
148-150 °C (lit.)
SMILES string
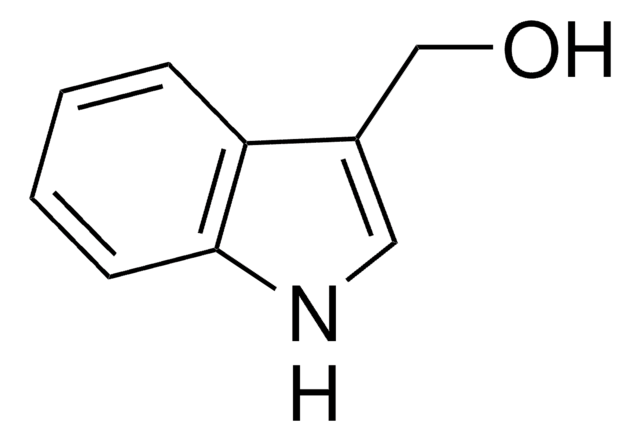
NC(=O)Cc1c[nH]c2ccccc12
InChI
1S/C10H10N2O/c11-10(13)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H2,11,13)
InChI key
ZOAMBXDOGPRZLP-UHFFFAOYSA-N
General description
Indole-3-acetamide is an auxin precursor.
Application
Indole-3-acetamide was used in the synthesis of [5.5.6.6]diazafenestrane skeleton and indole-3-acetic acid.
Reactant for the synthesis of:
- PET agent for imaging of protein kinase C
- A potential agent against Prion Disease
- Protein kinase C (PKC) inhibitor bisindolylmaleimide IV
- Glycogen synthase kinase-3ß (GSK-3ß) inhibitors
- Inhibitors of CaMKIId
- A VEGF inhibitor
- JAK3 inhibitors
- Inhibitors of NAD+-Dependent Histone Deacetylases
- Inhibitors of human adipocyte fatty acid-binding protein
- Cyclin-dependent kinase inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Elena Tsavkelova et al.
Fungal genetics and biology : FG & B, 49(1), 48-57 (2011-11-15)
The plant hormone indole-3-acetic acid (IAA) can be synthesized from tryptophan via the intermediate indole-3-acetamide (IAM). The two genes, IaaM (encoding tryptophan monooxygenase) and IaaH (encoding indole-3-acetamide hydrolase) that constitute the IAM pathway have been described in plant-associated bacteria. We
S Taliani et al.
Current medicinal chemistry, 16(26), 3359-3380 (2009-06-25)
The Translocator protein (TSPO), formerly known as the peripheral-type benzodiazepine receptor, is an 18 kDa mitochondrial protein primarily involved in steroid biosynthesis in both peripheral and glial cells. It has been extensively reported that TSPO regulates the rate-limiting translocation of
Atsushi Umehara et al.
Organic letters, 16(9), 2526-2529 (2014-04-24)
Total syntheses of leuconodine B, melodinine E, and leuconoxine were accomplished via a divergent route. The [5.5.6.6]diazafenestrane skeleton was constructed from an indole-3-acetamide derivative via DMDO oxidation to hydroxylindolenine, TMSOTf/2,6-lutidine mediated cyclic aminal formation, and diastereoseletive ring-closing metathesis of a
Daiana Duca et al.
Antonie van Leeuwenhoek, 106(1), 85-125 (2014-01-22)
Indole-3-acetic acid (IAA) is an important phytohormone with the capacity to control plant development in both beneficial and deleterious ways. The ability to synthesize IAA is an attribute that many bacteria including both plant growth-promoters and phytopathogens possess. There are
Christian O Dimkpa et al.
Applied and environmental microbiology, 78(5), 1404-1410 (2012-01-03)
The beneficial bacterium Pseudomonas chlororaphis O6 produces indole-3-acetic acid (IAA), a plant growth regulator. However, the pathway involved in IAA production in this bacterium has not been reported. In this paper we describe the involvement of the indole-3-acetamide (IAM) pathway
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service