244988
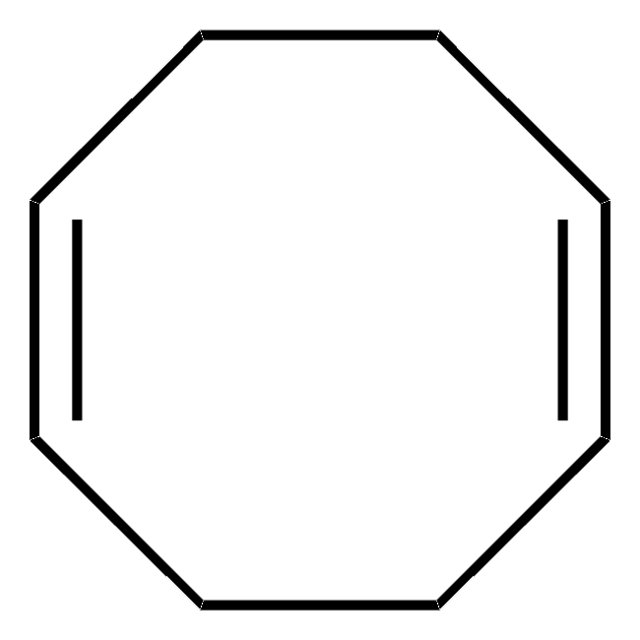
Bis(1,5-cyclooctadiene)nickel(0)
Synonym(s):
Bis(cyclooctadiene)nickel, Ni(COD)2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H24Ni
CAS Number:
Molecular Weight:
275.06
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
parameter
temperature sensitive
mp
60 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI key
JRTIUDXYIUKIIE-KZUMESAESA-N
Application
Catalyst for the cycloaddition of 1,3-dienes.
Reactant for:
Catalyst for:
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
Used to catalyze the addition of allyl phenyl sulfide to alkynes leading to 1,4-dienes. The reaction with terminal acetylenes proceeds in high yield and high selectivity. A variety of functional groups are tolerated.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
Target Organs
Lungs
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tsung-Han Lin et al.
ACS applied materials & interfaces, 9(5), 4948-4955 (2017-01-13)
The race for performance of integrated circuits is nowadays facing a downscale limitation. To overpass this nanoscale limit, modern transistors with complex geometries have flourished, allowing higher performance and energy efficiency. Accompanying this breakthrough, challenges toward high-performance devices have emerged
Journal of the American Chemical Society, 111, 6432-6432 (1989)
Addison N Desnoyer et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(20), 5259-5268 (2019-01-30)
The electronic nature of Ni π-complexes is underexplored even though these complexes have been widely postulated as intermediates in organometallic chemistry. Herein, the geometric and electronic structure of a series of nickel π-complexes, Ni(dtbpe)(X) (dtbpe=1,2-bis(di-tert-butyl)phosphinoethane; X=alkene or carbonyl containing π-ligands)
Qiang Gao et al.
Acta biomaterialia, 51, 112-124 (2017-01-31)
Numerous antimicrobial coatings have been developed for biomedical devices/implants, but few can simultaneously fulfill the requirements for antimicrobial and antifouling ability and biocompatibility. In this study, to develop an antimicrobial and antibiofilm surface coating, diblock amphiphilic molecules with antimicrobial and
Ruimao Hua et al.
Organic letters, 9(2), 263-266 (2007-01-16)
Allylic sulfides add to alkynes in the presence of nickel complexes efficiently to afford thio-1,4-dienes regio- and stereoselectively. Functional groups such as alkoxy, siloxy, hydroxy, carboalkoxy, chloro, and cyano groups are tolerated. A mechanism that involves a pi-allyl nickel intermediate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service