All Photos(3)
About This Item
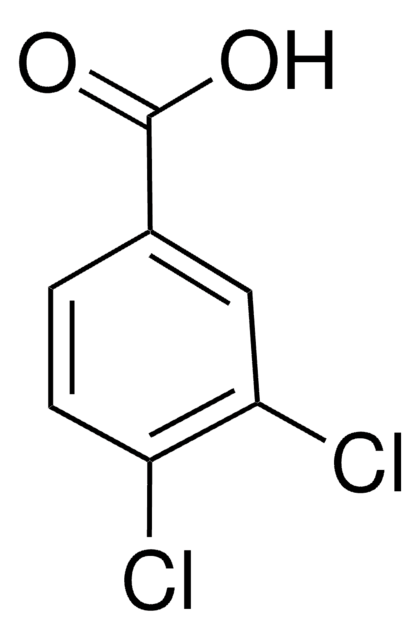
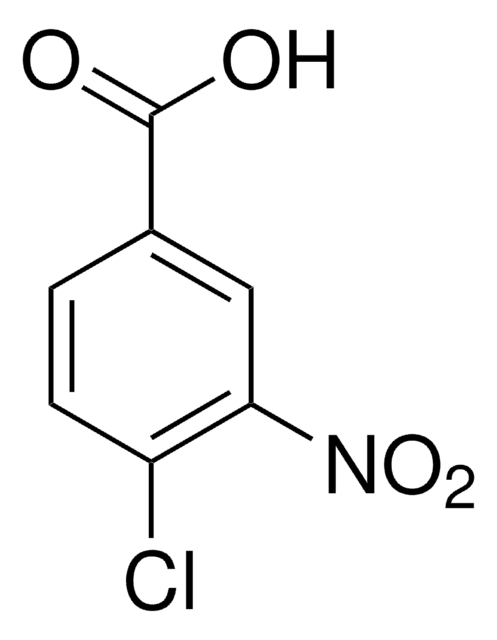
Linear Formula:
Cl2C6H3CO2H
CAS Number:
Molecular Weight:
191.01
Beilstein:
973353
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
301 °C (lit.)
mp
151-154 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1cc(Cl)ccc1Cl
InChI
1S/C7H4Cl2O2/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3H,(H,10,11)
InChI key
QVTQYSFCFOGITD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,5-Dichlorobenzoic acid is converted to 4-chlorocatechol by Pseudomonas sp. CPE2 strain.
Application
2,5-Dichlorobenzoic acid was used as calibration standard to investigate the gas-phase OH reaction products of biphenyl, monochlorobiphenyl and dichlorophenyl.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gas-phase oxidation products of biphenyl and polychlorinated biphenyls.
Brubaker WW and Hites RA.
Environmental Science & Technology, 32(24), 3913-3918 (1998)
D Di Gioia et al.
Research in microbiology, 149(5), 339-348 (1998-10-10)
Pyrocatechase activity was studied in the Pseudomonas sp. CPE2 strain, which is capable of growing on 2-chlorobenzoic and 2,5-dichlorobenzoic acid, giving rise to catechol and 4-chlorocatechol, respectively, as intermediate metabolites. The CPE2 crude extract was found to metabolize both catechol
Catherine J Duckett et al.
Rapid communications in mass spectrometry : RCM, 16(4), 245-247 (2002-01-30)
The use of directly coupled high performance liquid chromatography/inductively coupled plasma mass spectroscopy (HPLC/ICPMS) employing chlorine ((35)Cl/(37)Cl) detection has been investigated with respect to the detection and quantitation of the drugs diclofenac and chlorpromazine. By integration of peak areas in
V L Romanov et al.
Mikrobiologiia, 62(5), 887-896 (1993-09-01)
The strain Pseudomonas aeruginosa 142 isolated from the utilising PSBs bacterial association was capable of growth on 2-chloro- and 2,4-dichlorobenzoates as sole carbon sources, but it did not utilize 3-Cl, 4-Cl, 3,5-diCl- and 2,6-dichlorobenzoates. P. aeruginosa 142 dehalogenated 2-Cl-, 2,4-diCl-
B J van der Woude et al.
Biodegradation, 6(1), 39-46 (1995-01-01)
From long-term chemostat experiments, variants of Pseudomonas aeruginosa JB2 were obtained which exhibited altered properties with respect to the metabolism of 2,5-dichlorobenzoic acid (2,5-DBA). Thus, unlike the original strain JB2-WT, strain JB2-var1 is able to grow in continuous culture on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service