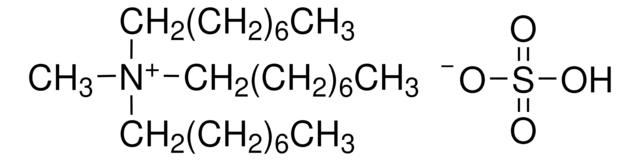70444
Tributylmethylammonium chloride
≥98.0% (T)
Synonym(s):
Methyltributylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)3N(Cl)CH3
CAS Number:
Molecular Weight:
235.84
Beilstein:
6300212
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
crystals
SMILES string
[Cl-].CCCC[N+](C)(CCCC)CCCC
InChI
1S/C13H30N.ClH/c1-5-8-11-14(4,12-9-6-2)13-10-7-3;/h5-13H2,1-4H3;1H/q+1;/p-1
InChI key
IPILPUZVTYHGIL-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Tributylmethylammonium chloride is a quaternary ammonium salt commonly used as a catalyst in the synthesis of ɛ-caprolactone and 1-substituted tetrazoles.
Application
Tributylmethylammonium chloride can be used as a phase transfer catalyst in the synthesis of ɛ-caprolactone by Baeyer-Villiger oxidation of cyclohexanone in the presence of KHSO5 as an oxidizing agent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New and efficient technique for the synthesis of ?-caprolactone using KHSO5 as an oxidising agent in the presence of a phase transfer catalyst
Baj S, et al
Applied Catalysis A: General, 395(1-2), 49-52 (2011)
One-pot synthesis of 1-substituted 1 H-1, 2, 3, 4-tetrazoles from 2aminothiazoles using tributylmethylammonium chloride as a catalyst
Nagaraju K, et al
Heterocyclic Communications, 23(5), 365-368 (2017)
Soon-Sun Hong et al.
Archives of pharmacal research, 29(4), 318-322 (2006-05-10)
Many quaternary ammonium salts are incompletely absorbed after their oral administration and may also be actively secreted into the intestine. However, the underlying mechanism(s) that control the transport of these cations across the intestinal epithelium is not well understood. In
I S Song et al.
American journal of physiology. Gastrointestinal and liver physiology, 281(2), G515-G525 (2001-07-12)
The objective of this study was to examine whether ion pair complexation with endogenous bile salts in hepatocytes contributes to the preferential biliary excretion of organic cations (OCs). Tributylmethylammonium (TBuMA; mol wt 200) and triethylmethylammonium (TEMA; mol wt 116) were
Moon Kyoung Kim et al.
Journal of pharmaceutical sciences, 94(12), 2644-2655 (2005-11-01)
The oral bioavailability of tributylmethyl ammonium (TBuMA), an organic cation (OC), exhibited a dose-dependency (i.e., 17, 27, and 35% at doses of 0.4, 4, or 12 micromol/kg, respectively) in the rat. Relevant mechanisms were investigated in the present study by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service