370207
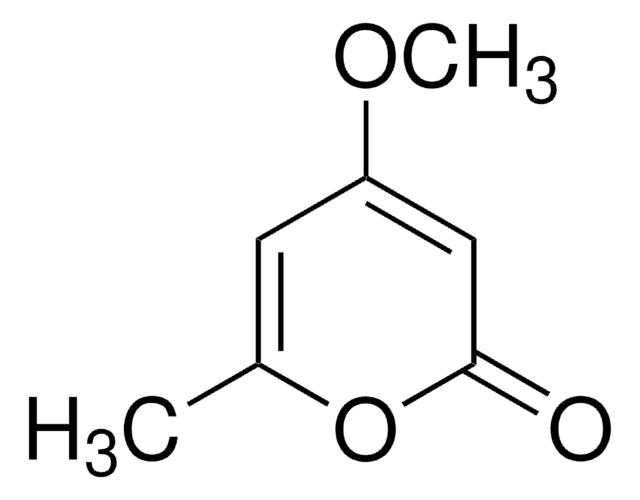
4,6-Dimethyl-α-pyrone
98%
Synonym(s):
4,6-Dimethyl-2H-pyran-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8O2
CAS Number:
Molecular Weight:
124.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
48-50 °C (lit.)
functional group
ester
SMILES string
CC1=CC(C)=CC(=O)O1
InChI
1S/C7H8O2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3
InChI key
IXYLIUKQQQXXON-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
General description
4,6-Dimethyl-α-pyrone was immobilized in a guanidinium-sulfonate-calixarene (G4C) crystalline network, which upon exposure to ultraviolet irradiation, transforms the entrapped 4,6-dimethyl-α-pyrone into a 4,6-dimethyl-β-lactone. Thin solid films layers of 4,6-dimethyl-α-pyrone on UV irradiation at -190°C were investigated.
Application
4,6-Dimethyl-α-pyrone (Me21) has been used in the preparation of G4C{Me21} via reacting with tetra-p-sulfocalix[4]arene (C) and guanidine hydrochloride (GCl). It may be used in the preparation of host-guest complex G4C{Me21} by reacting with crystalline guanidinium-sulfonate-calixarene (G4C). This complex on UV irradiation afforded 1,3-dimethylcyclobutadiene (Me2CBD).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Single-crystal X-ray structure of 1,3-dimethylcyclobutadiene by confinement in a crystalline matrix.
Yves-Marie Legrand et al.
Science (New York, N.Y.), 329(5989), 299-302 (2010-07-22)
Cyclobutadiene (CBD), the smallest cyclic hydrocarbon bearing conjugated double bonds, has long intrigued chemists on account of its strained geometry and electronic instability, but the parent compound and its unperturbed derivatives have thus far eluded crystallographic characterization. In this work
Yves-Marie Legrand et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(36), 10021-10028 (2011-09-08)
Cyclobutadiene (CBD), the smallest cyclic hydrocarbon bearing conjugated double bonds, has long intrigued chemists because of its chemical characteristics. The question of whether the molecule could be prepared at all has been answered, but the parent compound and its unperturbed
4, 6-Dimethyl-a-pyrone: a matrix isolation study of the photochemical generation of conjugated ketene, Dewar valence isomer and 1, 3-dimethyl-cyclobutadiene.
Breda S, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 162(1), 139-151 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service