All Photos(1)
About This Item
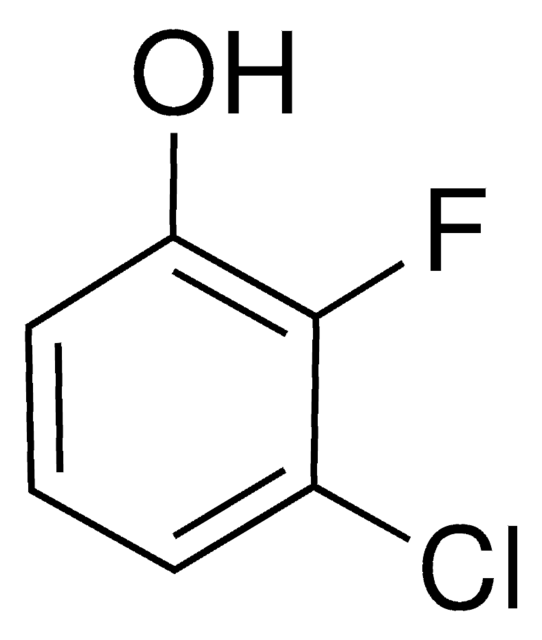
Linear Formula:
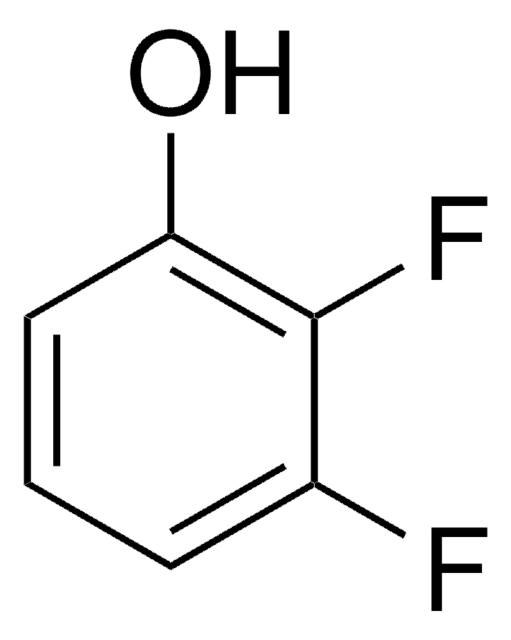
F2C6H3OH
CAS Number:
Molecular Weight:
130.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
40-42 °C (lit.)
functional group
fluoro
SMILES string
Oc1cc(F)ccc1F
InChI
1S/C6H4F2O/c7-4-1-2-5(8)6(9)3-4/h1-3,9H
InChI key
INXKVYFOWNAVMU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Conversion of 2,5-difluorophenol by whole cells of Rhodococcus opacus 1G has been investigated by 9F NMR analysis.
Application
2,5-Difluorophenol has been used in the synthesis of di- or trifluorinated hydroxybenzoic acids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
127.4 °F - closed cup
Flash Point(C)
53 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The regioexhaustive functionalization of difluorophenols and trifluorophenols through organometallic intermediates.
Marzi E, et al.
Synthesis, 2004(10), 1609-1618 (2004)
V S Bondar et al.
FEMS microbiology letters, 181(1), 73-82 (1999-11-24)
The regiospecificity of hydroxylation of C2-halogenated phenols by Rhodococcus opacus 1G was investigated. Oxidative defluorination at the C2 position ortho with respect to the hydroxyl moiety was preferred over hydroxylation at the non-fluorinated C6 position for all 2-fluorophenol compounds studied.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service