288365
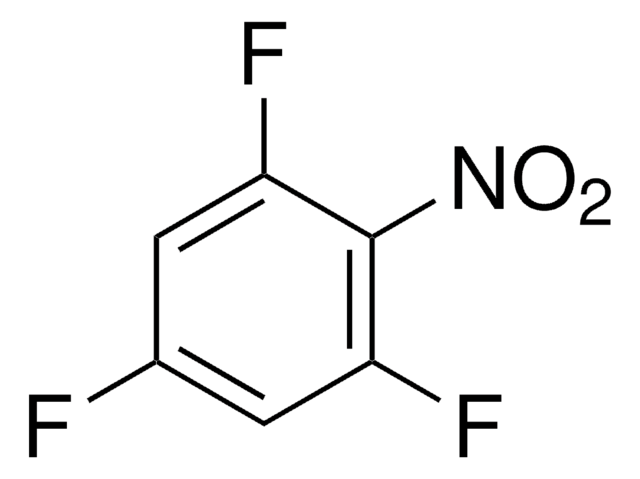
3,4-Difluoronitrobenzene
99%
Synonym(s):
1,2-Difluoro-4-nitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H3NO2
CAS Number:
Molecular Weight:
159.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.509 (lit.)
bp
76-80 °C/11 mmHg (lit.)
density
1.437 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
[O-][N+](=O)c1ccc(F)c(F)c1
InChI
1S/C6H3F2NO2/c7-5-2-1-4(9(10)11)3-6(5)8/h1-3H
InChI key
RUBQQRMAWLSCCJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The experimental and computational thermochemical study of 3,4-difluoronitrobenzene was studied.
Application
3,4-Difluoronitrobenzene was used in the preparation of xanthones and acridones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oludotun A Phillips et al.
Journal of enzyme inhibition and medicinal chemistry, 35(1), 1471-1482 (2020-07-09)
Oxazolidinone hydroxamic acid derivatives were synthesised and evaluated for inhibitory activity against leukotriene (LT) biosynthesis in three in vitro cell-based test systems and on direct inhibition of recombinant human 5-lipoxygenase (5-LO). Thirteen of the 19 compounds synthesised were considered active ((50%
Synthesis of heterocyclic compounds via nucleophilic aroylation catalyzed by imidazolidenyl carbene.
Yumiko Suzuki et al.
Chemical & pharmaceutical bulletin, 54(12), 1653-1658 (2006-12-02)
Xanthones and acridones were synthesized from 3,4-difluoronitrobenzene and 2-fluorobenzaldehydes in two or three steps. The key step was nucleophilic aroylation catalyzed by imidazolidenyl carbene. The nucleophilic aroylation of 3,4-difluoronitrobenzene afforded 2,2'-difluoro-4-nitrobenzophenones. The cyclization of the difluorobenzophenones with O-nucleophile and N-nucleophile
Manuel A V Ribeiro da Silva et al.
The journal of physical chemistry. B, 114(40), 12914-12925 (2010-09-24)
This work reports the experimental and computational thermochemical study performed on three difluorinated nitrobenzene isomers: 2,4-difluoronitrobenzene (2,4-DFNB), 2,5-difluoronitrobenzene (2,5-DFNB), and 3,4-difluoronitrobenzene (3,4-DFNB). The standard (p° = 0.1 MPa) molar enthalpies of formation in the liquid phase of these compounds were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service