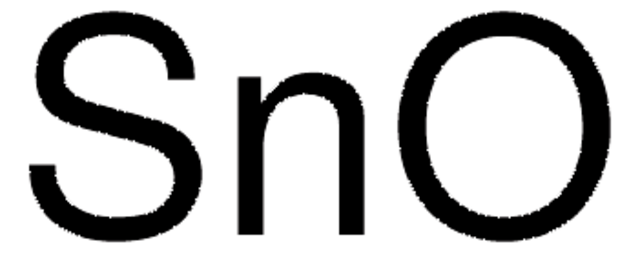244651
Tin(IV) oxide
−325 mesh, 99.9% trace metals basis
Synonym(s):
Stannic oxide
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
SnO2
CAS Number:
Molecular Weight:
150.71
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
powder
particle size
−325 mesh
density
6.95 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Sn]=O
InChI
1S/2O.Sn
InChI key
XOLBLPGZBRYERU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tin(IV) oxide (SnO2) is an n-type wide band gap semiconductor with high transmittance at nearIR and visible region. It is scratch resistant and chemically inert.
Application
Tin(IV) oxide has been used to prepare thin films of TiO2-doped SnO2 oxide nanocomposites.
It can be used as astarting material to prepare niobium and zinc-doped titanium-tin-oxidesolid-solution ceramics, which are applicable in the field of electronicdevices.
It can be used as astarting material to prepare niobium and zinc-doped titanium-tin-oxidesolid-solution ceramics, which are applicable in the field of electronicdevices.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lina Gao et al.
Langmuir : the ACS journal of surfaces and colloids, 29(3), 957-964 (2012-12-25)
As advanced electrodes for direct alcohol fuel cells, graphene-Pd and graphene-Pt composites with a trace of SnO(2) have been successfully synthesized by a modified electroless plating technique. The surface of graphene oxide is first sensitized by Sn(2+) ions, and subsequently
Linlin Li et al.
Nanoscale, 5(1), 134-138 (2012-11-14)
Novel eggroll-like CaSnO(3) nanotubes have been prepared by a single spinneret electrospinning method followed by calcination in air for the first time. The electrospun sample as a lithium-ion battery electrode material exhibited improved cycling stability and rate capability by virtue
Yinzhu Jiang et al.
ACS applied materials & interfaces, 4(11), 6216-6220 (2012-10-31)
Porous SnO₂/graphene composite thin films are prepared as anodes for lithium ion batteries by the electrostatic spray deposition technique. Reticular-structured SnO₂ is formed on both the nickel foam substrate and the surface of graphene sheets according to the scanning electron
Ilsun Yoon et al.
Nanoscale, 5(2), 552-555 (2012-12-13)
Here we demonstrate a facile method of quantifying the decaying optical field surrounding free-standing tin dioxide (SnO(2)) nanofiber waveguides. Through the use of thin self-assembled polyelectrolyte coatings and fluorescent optical transmitters we map out the optical intensity as a function
Junfei Liang et al.
ACS applied materials & interfaces, 4(11), 5742-5748 (2012-10-24)
A flexible free-standing graphene/SnO₂ nanocomposites paper (GSP) was prepared by coupling a simple filtration method and a thermal reduction together for the first time. Compared with the pure SnO₂ nanoparticles, the GSP exhibited a better cycling stability, because the graphene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service