235202
4-Bromomethyl-7-methoxycoumarin
97%
Synonym(s):
BMC, Br-Mmc
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
mp
213-215 °C (lit.)
solubility
chloroform: soluble 20 mg/mL, clear, colorless to faintly yellow
fluorescence
λex 322 nm; λem 395 nm (after derivatization)
functional group
bromo
ester
SMILES string
COc1ccc2C(CBr)=CC(=O)Oc2c1
InChI
1S/C11H9BrO3/c1-14-8-2-3-9-7(6-12)4-11(13)15-10(9)5-8/h2-5H,6H2,1H3
InChI key
CTENSLORRMFPDH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as derivatization reagent in determination of 5-fluorouracil (5-FU) in human plasma by HPLC-tandem mass spectrometry
- as derivatization reagent in fluorescence detection of sodium monofluoroacetate (MFA-Na) in biological samples by HPLC
- in the synthesis of new caged derivatives of hydrolysis-resistant 8-bromoadenosine cyclic 3′,5′-monophosphate (8-Br-cAMP) and 8-bromoguanosine cyclic 3′,5′-monophosphate (8-Br-cGMP)
- in TLC and HPLC separation and fluorometric analysis of a wide range of naturally occurring acids, including bile and thromboxane B2
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service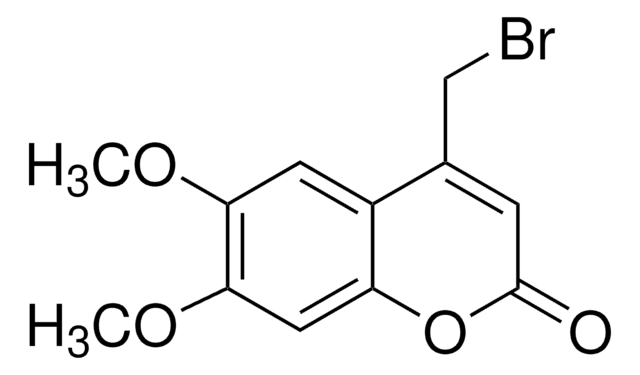

![Tris[2-(2-methoxyethoxy)ethyl]amine 95%](/deepweb/assets/sigmaaldrich/product/structures/266/084/fe6f96ea-6320-4c87-8438-fa06ddcadd83/640/fe6f96ea-6320-4c87-8438-fa06ddcadd83.png)