All Photos(3)
About This Item
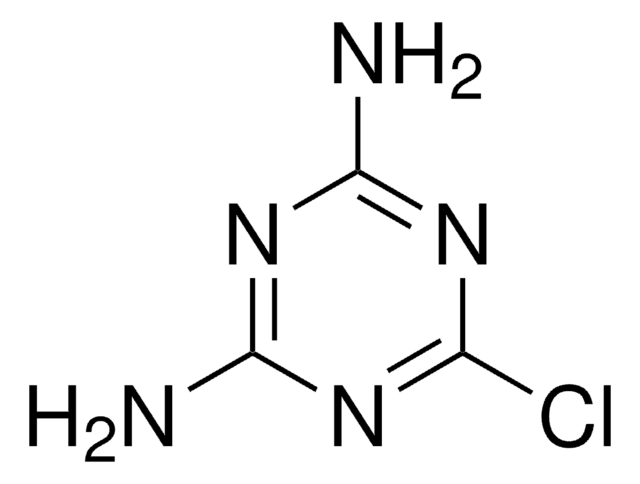
Empirical Formula (Hill Notation):
C4H3Cl2N3O
CAS Number:
Molecular Weight:
179.99
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
bp
132-134 °C/49 mmHg (lit.)
mp
86-88 °C (lit.)
SMILES string
COc1nc(Cl)nc(Cl)n1
InChI
1S/C4H3Cl2N3O/c1-10-4-8-2(5)7-3(6)9-4/h1H3
InChI key
JKAPWXKZLYJQJJ-UHFFFAOYSA-N
Application
2,4-Dichloro-6-methoxy-1,3,5-triazine was used as reagent in radioimmunoassay for D-Ala2-Dermorphin, a natural peptide extracted from amphibian skin. It was also used in the preparation of:
- α-cyclodextrin [2]-rotaxanes
- substituted s-triazines
- series of chiral derivatizing reagents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L Negri et al.
Peptides, 2 Suppl 2, 45-49 (1981-01-01)
A selective RIA for D-Ala2-Dermorphin (Der), a natural peptide extracted from amphibian skin, has been developed using an antibody raised in rabbits against Der which has been coupled to BSA through its phenolic hydroxyl groups of tyrosine residues with 2,4-Dichloro-6-methoxy-1,3,5-triazine.
Ryan E Dawson et al.
Organic & biomolecular chemistry, 6(10), 1814-1821 (2008-05-03)
Ten alpha-cyclodextrin [2]-rotaxanes have been prepared with alkane-, stilbene- and azobenzene-based axles, capped through nucleophilic substitution of either 2-chloro-4,6-dimethoxy-1,3,5-triazine or 2,4-dichloro-6-methoxy-1,3,5-triazine in aqueous solution, followed by further substitution of the remaining triazinyl chlorine in some cases when the latter capping
Recent applications of 2, 4, 6-trichloro-1, 3, 5-triazine and its derivatives in organic synthesis.
Blotny G.
Tetrahedron, 62(41), 9507-9522 (2006)
H Brückner et al.
Journal of chromatography. A, 998(1-2), 73-82 (2003-07-17)
A series of chiral derivatizing reagents (CDRs) was synthesized by nucleophilic replacement of one chlorine atom in cyanuric chloride (2,4,6-trichloro-1,3,5-triazine; s-triazine) by alkoxy (methoxy, butoxy, 1,1,1-trifluoroethoxy) or aryloxy groups (phenoxy, nitrophenoxy, phenylphenoxy, 4-methylcoumaryloxy), and displacement of a second chlorine by
Tatsuki Nakano et al.
Analytica chimica acta, 880, 145-151 (2015-06-21)
In this study, a novel pre-column excimer fluorescence derivatization reagent, 2-chloro-4-methoxy-6-(4-(pyren-4-yl)butoxy)-1,3,5-triazine (CMPT), was developed for polyamines, specifically histamine. By CMPT derivatization, the polyamines, histamine and tyramine were converted to polypyrene derivatives, and emitted intra-molecular excimer fluorescence at 475nm. This could
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service