135585
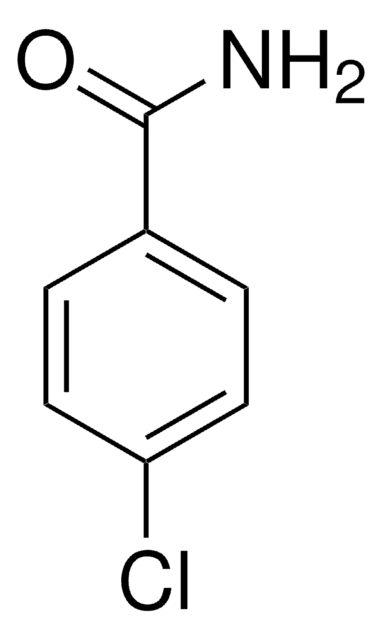
4-Chlorobenzoic acid
99%
Synonym(s):
4-CBA, p-Chlorobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
ClC6H4CO2H
CAS Number:
Molecular Weight:
156.57
Beilstein:
907196
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
238-241 °C (lit.)
solubility
methanol: soluble 1%, clear, colorless to faintly yellow
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccc(Cl)cc1
InChI
1S/C7H5ClO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)
InChI key
XRHGYUZYPHTUJZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Chlorobenzoic acid is a degradation product of indomethacin. It is degraded by Acinetobacter sp. strain ST-1 and causes its dehalogenation to yield 4-hydroxybenzoic acid under both aerobic and anaerobic conditions.
Application
4-Chlorobenzoic acid can be used:
- As a ligand to synthesize luminescent lanthanide complexes for bio-labeling or fiber communication applications.
- To prepare organotin(IV) chlorobenzoates exhibiting anticorrosion properties.
- As a ligand to synthesize di-n-butyl(4-chlorobenzoxy)(4-chlorobenzohydroxamato)tin(IV).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
460.4 °F - closed cup
Flash Point(C)
238 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and crystal structure of a mixed-ligand compound di-n-butyl (4-chlorobenzoxy)(4-chlorobenzohydroxamato) tin (IV)
Shang X, et al.
Journal of Organometallic Chemistry, 690(17), 3997-4000 (2005)
Mariana Lanzarini-Lopes et al.
Chemosphere, 188, 304-311 (2017-09-10)
Electrochemical oxidation (EO) is an advanced oxidation process for water treatment to mineralize organic contaminants. While proven to degrade a range of emerging pollutants in water, less attention has been given to quantify the effect of operational variables such applied
Aliyeh Hasanzadeh et al.
Scientific reports, 9(1), 13780-13780 (2019-09-26)
Nanocarbon materials are considered to be active for electrochemical oxygen reduction reaction (ORR) for hydrogen peroxide (H2O2) synthesis. In the present work, a new type of fullerene 60 (C60)-carbon nanotubes (CNTs) hybrid with covalently attached C60 onto outer surface of
The Synthesis, characterization and comparative anticorrosion study of some organotin (IV) 4-chlorobenzoates
Kurniasih H, et al.
Orient. J. Chem., 31(4), 2377-2383 (2015)
Ruiyang Xiao et al.
Environmental pollution (Barking, Essex : 1987), 257, 113498-113498 (2019-11-26)
Carbamazepine (CBZ), a widely detected pharmaceutical in wastewaters, cannot currently be treated by conventional activated sludge technologies, as it is highly resistant to biodegradation. In this study, the degradation kinetics and reaction mechanisms of CBZ by hydroxyl radical (OH) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service