130176
4-Nitrobenzaldehyde
98% (GC)
Synonym(s):
p-Nitrobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
O2NC6H4CHO
CAS Number:
Molecular Weight:
151.12
Beilstein:
386796
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98% (GC)
mp
103-106 °C (lit.)
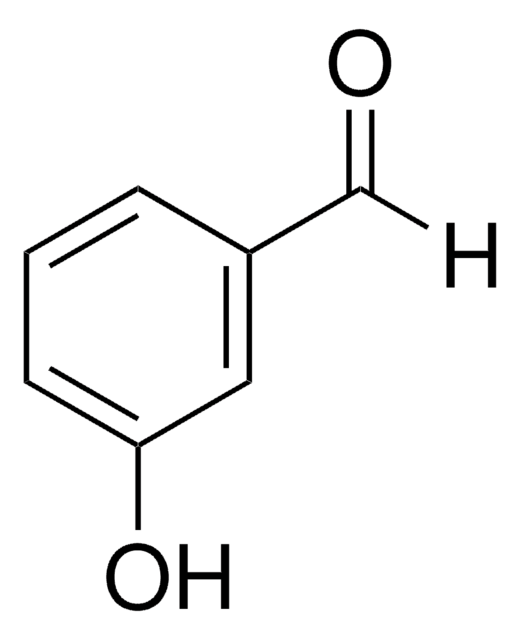
SMILES string
[O-][N+](=O)c1ccc(C=O)cc1
InChI
1S/C7H5NO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5H
InChI key
BXRFQSNOROATLV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Nitrobenzaldehyde was used in the preparation of homoallylic alcohols. It was also used to develop and evaluate a series of tripeptide organocatalysts.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Niels J M Pijnenburg et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(15), 4858-4868 (2013-02-26)
This paper describes a mechanistic study of the SCS-pincer Pd(II)-catalyzed auto-tandem reaction consisting of the stannylation of cinnamyl chloride with hexamethylditin, followed by an electrophilic allylic substitution of the primary tandem-reaction product with 4-nitrobenzaldehyde to yield homoallylic alcohols as the
Saadi Bayat et al.
Chirality, 25(11), 726-734 (2013-08-24)
A series of tripeptide organocatalysts containing a secondary amine group and two amino acids with polar side chain units were developed and evaluated in the direct asymmetric intermolecular aldol reaction of 4-nitrobenzaldehyde and cyclohexanone. The effectiveness of short polar peptides
H Maheswaran et al.
Chemical communications (Cambridge, England), (39)(39), 4066-4068 (2006-10-07)
The dichloro[(-)-sparteine-N,N']copper(II) complex provides Henry adducts with high enantioselectivities (73-97% ee) in Henry reaction between nitromethane and various aldehydes.
Chao Li et al.
Journal of biotechnology, 150(4), 539-545 (2010-10-21)
Several proteases, especially pepsin, were observed to directly catalyze asymmetric aldol reactions. Pepsin, which displays well-documented proteolytic activity under acidic conditions, exhibited distinct catalytic activity in a crossed aldol reaction between acetone and 4-nitrobenzaldehyde with high yield and moderate enantioselectivity.
Gang Wu et al.
The journal of physical chemistry. A, 112(5), 1024-1032 (2008-01-16)
We have used solid-state 17O NMR experiments to determine the 17O quadrupole coupling (QC) tensor and chemical shift (CS) tensor for the carbonyl oxygen in p-nitro-[1-(17)O]benzaldehyde. Analyses of solid-state 17O NMR spectra obtained at 11.75 and 21.15 T under both
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service