All Photos(2)
About This Item
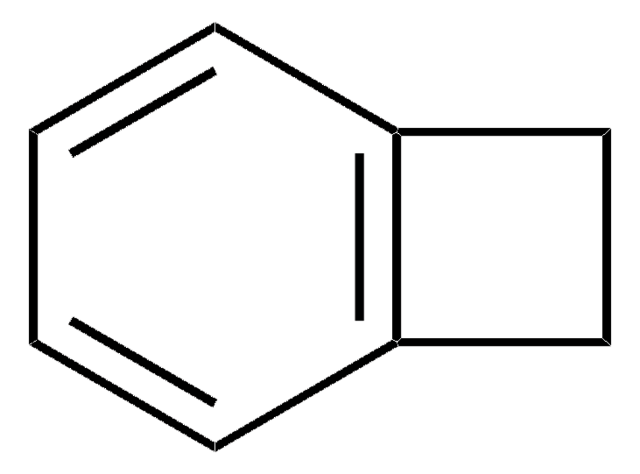
Empirical Formula (Hill Notation):
C8H4Cl2N2
CAS Number:
Molecular Weight:
199.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
160-162 °C (lit.)
functional group
chloro
storage temp.
−20°C
SMILES string
Clc1nnc(Cl)c2ccccc12
InChI
1S/C8H4Cl2N2/c9-7-5-3-1-2-4-6(5)8(10)12-11-7/h1-4H
InChI key
ODCNAEMHGMYADO-UHFFFAOYSA-N
Related Categories
Application
1,4-Dichlorophthalazine was used as starting reagent in the synthesis of series of 4-aryl-1-(4-methylpiperazin-1-yl)phthalazines. It was used as coupling reagent in the synthesis of novel soluble polymer-bound ligand. It was often used as building block in medicinal chemistry synthesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Matthew A J Duncton et al.
Bioorganic & medicinal chemistry letters, 16(6), 1579-1581 (2006-01-03)
A novel class of 1-(isoquinolin-5-yl)-4-arylamino-phthalazines is described as inhibitors of vascular endothelial growth factor receptor II (VEGFR-2). Many compounds display VEGFR-2 inhibitory activity with an IC(50) as low as 0.017 microM in an HTRF enzymatic assay. The compounds also inhibit
K De Wael et al.
Science & justice : journal of the Forensic Science Society, 55(6), 422-430 (2015-12-15)
Reactively-dyed black, navy blue and medium red cotton samples showing metamerism under fluorescent tube illumination were examined. Optical microscopy (bright field, polarization and fluorescence microscopy) was used, followed by microspectrometry in the visible range (MSP Vis), to differentiate the samples
A simple and effective soluble polymer-bound ligand for the asymmetric dihydroxylation of olefins: DHQD-PHAL-OPEG-OMe.
Kuang YQ, et al.
Tetrahedron Letters, 42(34), 5925-5927 (2001)
Synthesis of 4-aryl-1-(4-methylpiperazin-1-yl) phthalazines by Suzuki-type cross-coupling reaction.
Guery S, et al.
Synthesis, 2001(05), 699-701 (2001)
William H Bunnelle et al.
Journal of medicinal chemistry, 50(15), 3627-3644 (2007-06-26)
A series of exceptionally potent agonists at neuronal nicotinic acetylcholine receptors (nAChRs) has been investigated. Several N-(3-pyridinyl) derivatives of bridged bicyclic diamines exhibit double-digit-picomolar binding affinities for the alpha 4 beta 2 subtype, placing them with epibatidine among the most
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service