108928
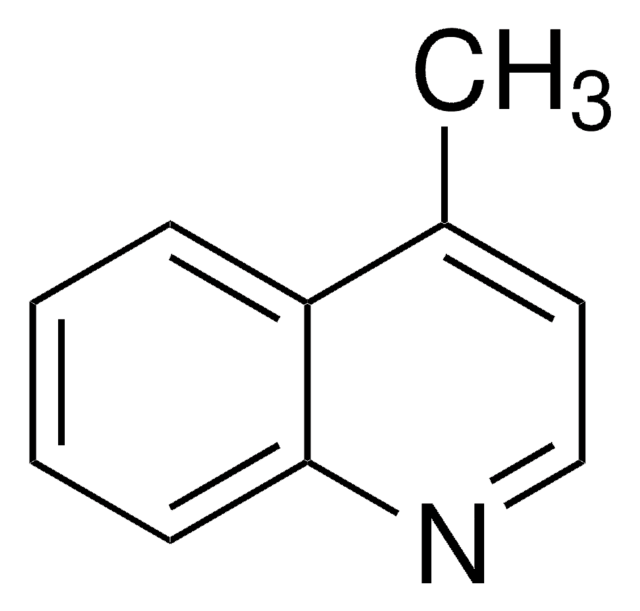
6-Methylquinoline
98%
Synonym(s):
p-Toluquinoline, NSC 4152
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H9N
CAS Number:
Molecular Weight:
143.19
Beilstein:
110336
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39180204
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
Assay
98%
refractive index
n20/D 1.614 (lit.)
bp
256-260 °C (lit.)
density
1.067 g/mL at 20 °C (lit.)
SMILES string
Cc1ccc2ncccc2c1
InChI
1S/C10H9N/c1-8-4-5-10-9(7-8)3-2-6-11-10/h2-7H,1H3
InChI key
LUYISICIYVKBTA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Methylquinoline can be used as primary carbon source in culture of Pseudomonas putida QP1. 6-Methylquinoline was used in the synthesis of fluorescent halide-sensitive quinolinium dyes and fluorescent probes for determination of chloride in biological systems.
Biochem/physiol Actions
6-Methylquinoline undergoes biodegradation by quinoline-degrading culture of Pseudomonas putida.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chloride sensitive probes for biological applications.
Geddes CD, et al.
Dyes and Pigments, 48(3), 227-231 (2001)
S Rothenburger et al.
Applied and environmental microbiology, 59(7), 2139-2144 (1993-07-01)
Selective culturing of pseudomonads that could degrade quinoline led to enrichment cultures and pure cultures with expanded substrate utilization and transformation capabilities for substituted quinolines in immobilized and batch cultures. Immobilized cells of the pseudomonad cultures rapidly transformed quinolines to
Umar Farooq Rizvi et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 10), o547-o549 (2008-10-08)
Molecules of (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-iodo-2-thienyl)prop-2-en-1-one, C(17)H(11)ClINOS, (I), and (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-methyl-2-furyl)prop-2-en-1-one, C(18)H(14)ClNO(2), (II), adopt conformations slightly twisted from coplanarity. Both structures are devoid of classical hydrogen bonds. However, nonclassical C-H...O/N interactions [with C...O = 3.146 (5) A and C...N = 3.487 (3) A] link
C D Geddes et al.
Analytical biochemistry, 293(1), 60-66 (2001-05-25)
Three fluorescent halide-sensitive quinolinium dyes have been produced by the reaction of the 6-methylquinoline heterocyclic nitrogen base with methyl bromide, methyl iodide, and 3-bromo-1-propanol. The quaternary salts, unlike the precursor molecule, are readily water soluble and the fluorescence intensity of
C E Scharping et al.
Carcinogenesis, 14(5), 1041-1047 (1993-05-01)
The hepatic microsomal metabolism of the carcinogenic 8-methylquinoline (8MQ) and its noncarcinogenic isomer, 6-methylquinoline (6MQ), were compared for preparations from control rats and rats pretreated with phenobarbital or 3-methylcholanthrene. For each compound the alcohol was the major metabolite, constituting 50-75%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service