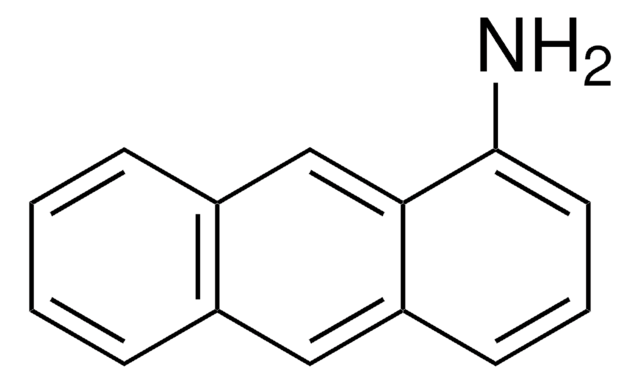
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H11N
CAS Number:
Molecular Weight:
193.24
Beilstein:
2209414
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
powder
mp
238-241 °C (lit.)
SMILES string
Nc1ccc2cc3ccccc3cc2c1
InChI
1S/C14H11N/c15-14-6-5-12-7-10-3-1-2-4-11(10)8-13(12)9-14/h1-9H,15H2
InChI key
YCSBALJAGZKWFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Aminoanthracene can be used as a reactant to prepare:
- Steroid derived naphthoquinoline asphaltene compounds via multicomponent cyclocondensation reaction with 5-α-cholestan-3-one and aromatic aldehydes.
- Naphtho[2,3- f ]quinoline derivatives by reacting with aromatic aldehyde and acetone or acetophenone catalyzed by iodine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An efficient method for the synthesis of naphthoquinoline derivatives catalyzed by iodine
Wang W, et al.
Heterocyclic Communications, 18(1), 17-21 (2012)
Steroid-Derived Naphthoquinoline Asphaltene Model Compounds: Hydriodic Acid Is the Active Catalyst in I2-Promoted Multicomponent Cyclocondensation Reactions
Schulze M, et al.
Organic Letters, 17(23), 5930-5933 (2015)
A Di Sotto et al.
Journal of ethnopharmacology, 127(3), 731-736 (2009-12-09)
The aerial parts of Sisymbrium officinale Scop. are commonly used to treat airway ailments, moreover in antiquity the herbal drug was reputed to possess anticancer properties. The results obtained in present work support the traditional use and the properties ascribed
Rodrigo Juliano Oliveira et al.
Toxicology in vitro : an international journal published in association with BIBRA, 20(7), 1225-1233 (2006-05-24)
Due to the need to identify new antimutagenic agents and to determine their mechanism of action, the present study examined the mechanism of action of the beta-glucan with regard to antimutagenicity using the micronucleus assay in CHO-k1 and HTC cell
Katalin Jemnitz et al.
Mutagenesis, 19(3), 245-250 (2004-05-05)
We studied the replacement of hepatic S9 with in vivo and in vitro induced hepatocytes as a metabolic activation system with the aim of broadening the possibilities of mutagenic assays. Rats were pretreated with beta-naphthoflavone (BNF), phenobarbital (PB), 3-methylcholanthrene (MC)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)