900761
Sodium 4-methylpyridine-2-sulfinate
≥95%
Synonym(s):
Willis 4-methyl pyridine-2-sulfinate
About This Item
Recommended Products
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: C-C Bond Formation
mp
342 °C
Application
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Description: Professor Willis and partners at Pfizer have shown with pyridine-2- sulfinates a palladium-catalyzed desulfinylative cross-coupling process can be realized.
Description: Professor Willis and partners at Pfizer have shown with pyridine-2- sulfinates a palladium-catalyzed desulfinylative cross-coupling process can be realized.
Description: Professor Willis and partners at Pfizer have shown with pyridine-2- sulfinates a palladium-catalyzed desulfinylative cross-coupling process can be realized.
Description: Professor Willis and partners at Pfizer have shown with pyridine-2- sulfinates a palladium-catalyzed desulfinylative cross-coupling process can be realized.
Related Content
Research in the Willis group is focused on the development of new catalysts and reactions for synthetic chemistry. The group is particularly interested in addressing synthetic challenges that are applicable to the pharmaceutical and agrochemical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service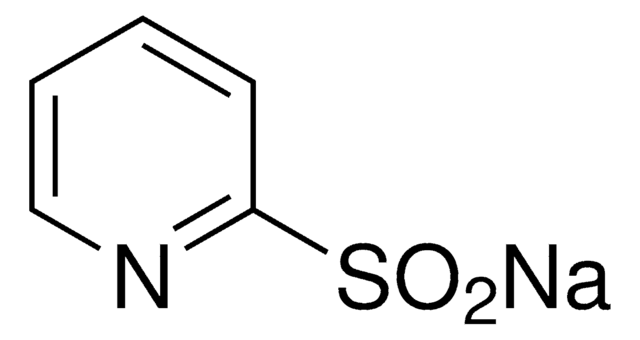
![1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)