37870
3-(2,4-Dihydroxyphenyl)propionic acid
≥95.0% (HPLC)
Synonym(s):
2,4-Dihydroxyhydrocinnamic acid, Hydroumbellic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H10O4
CAS Number:
Molecular Weight:
182.17
Beilstein:
2694769
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (HPLC)
form
solid
mp
158-162 °C
SMILES string
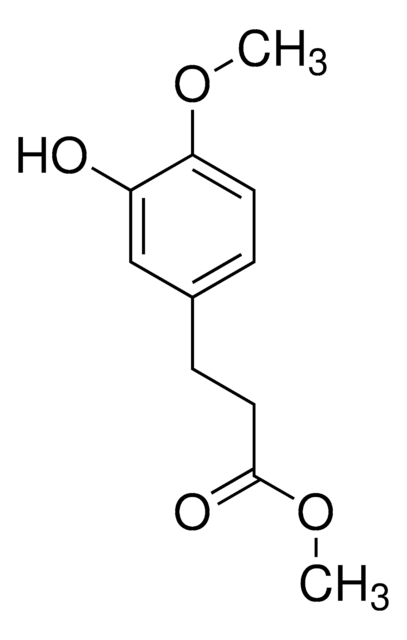
OC(=O)CCc1ccc(O)cc1O
InChI
1S/C9H10O4/c10-7-3-1-6(8(11)5-7)2-4-9(12)13/h1,3,5,10-11H,2,4H2,(H,12,13)
InChI key
HMCMTJPPXSGYJY-UHFFFAOYSA-N
General description
3-(2,4-Dihydroxyphenyl)propionic acid is a 3,4-dihydroxyphenylalanine (DOPA) analog. It is a building block for heterocyclic compounds.
Application
3-(2,4-Dihydroxyphenyl)propionic acid (DHPPA) may be used as substrate for the o-diphenolase in microplate assays of serum and hemocyte supernatants from QXR3 and wild type oysters for the quantitation of o-diphenolase activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A new synthesis of psoralene [(author's transl)].
O Dann et al.
Archiv der Pharmazie, 308(2), 121-130 (1975-02-01)
B Morin et al.
The Biochemical journal, 330 ( Pt 3), 1059-1067 (1998-05-23)
A major product of hydroxy-radical addition to tyrosine is 3, 4-dihydroxyphenylalanine (DOPA) which has reducing properties. Protein-bound DOPA (PB-DOPA) has been shown to be a major component of the stable reducing species formed during protein oxidation under several conditions. The
Kathryn Newton et al.
Developmental and comparative immunology, 28(6), 565-569 (2004-06-05)
QX is a fatal disease in Sydney rock oysters (Saccostrea glomerata) that results from infection by the protistan parasite, Marteilia sydneyi. Since 1997, the New South Wales Fisheries Service has bred S. glomerata for resistance to QX disease. The current
Violetta Mohos et al.
Biomolecules, 10(3) (2020-03-12)
Flavonoids are abundant polyphenols in nature. They are extensively biotransformed in enterocytes and hepatocytes, where conjugated (methyl, sulfate, and glucuronide) metabolites are formed. However, bacterial microflora in the human intestines also metabolize flavonoids, resulting in the production of smaller phenolic
Violetta Mohos et al.
International journal of molecular sciences, 20(11) (2019-06-05)
Quercetin is an abundant flavonoid in nature and is used in several dietary supplements. Although quercetin is extensively metabolized by human enzymes and the colonic microflora, we have only few data regarding the pharmacokinetic interactions of its metabolites. Therefore, we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service