37640
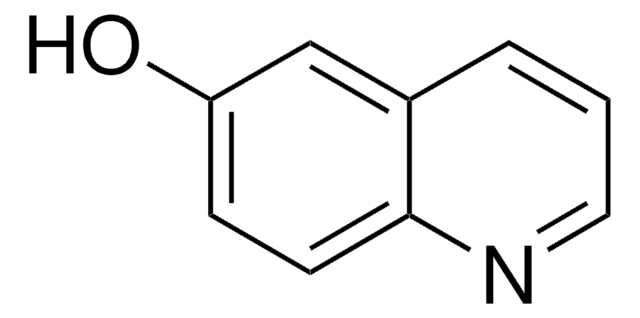
2,8-Quinolinediol
≥99.0% (HPLC)
Synonym(s):
2,8-Dihydroxyquinoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein:
472906
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (HPLC)
form
powder
mp
~290 °C (dec.)
SMILES string
Oc1ccc2cccc(O)c2n1
InChI
1S/C9H7NO2/c11-7-3-1-2-6-4-5-8(12)10-9(6)7/h1-5,11H,(H,10,12)
InChI key
ZXZKYYHTWHJHFT-UHFFFAOYSA-N
General description
2,8-Quinolinediol is a quinolone derivative. It has been reported as metabolite of 8-hydroxyquinoline-N-oxide in rabbits. Synthesis of 2,8-quinolinediol has been reported. It is reported as one of the six possible forms of 8-hydroxycarbostyril. It has been detected as new UV-absorbing compound (UAC) in cow milk and its structure was elucidated using HRMS and by 1H, 13C and 1H ×13C NMR.It is also known as 8-hydroxycarbostyril or 8-hydroxyquinolin-2(1H)-one.
8-hydroxyquinolin-2(1H)-one has been reported as the tautomeric form of 2,8-quinolinediol. 2,8-Quinolinediol (2,8-Dihydroxyquinoline) has been identified as one of the metabolite of quinolone formed in the culture medium of gram-negative bacteria, Pseudomonas stutzeri.
Application
2,8-Quinolinediol is suitable for use as standard in a study to identify the urinary metabolites for the toxicity related processes and pathogenesis induced by doxorubicin (DOX) to rats by online and off-line LC-MS techniques. It may be used as starting reagent for the preparation of two powerful β2-adrenergic receptor agonists, used for the treatment of asthma:
- Procaterol
- Indacaterol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Muhammad Bilal et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 183, 417-424 (2017-05-06)
A green tunable dispersive liquid-liquid micro extraction (TDLLME) technique was established for the simultaneous enrichment of lead (Pb) and cadmium (Cd) from different lakes water before analysis by flame atomic absorption spectrometry (FAAS). A solvent known as tunable polarity solvent
Jiangshan Wang et al.
Metabolomics : Official journal of the Metabolomic Society, 5(4), 407-418 (2010-01-05)
A metabolomics-based systems toxicology approach was used to profile the urinary metabolites for the toxicity related processes and pathogenesis induced by doxorubicin (DOX) to rats. Endogenous metabolite profiles were obtained with ultra performance liquid chromatography-mass spectrometry (UPLC-MS) for rats receiving
O P Shukla
Applied and environmental microbiology, 51(6), 1332-1342 (1986-06-01)
A Pseudomonas sp. isolated from sewage by enrichment culture on quinoline metabolized this substrate by a novel pathway involving 8-hydroxycoumarin. During early growth of the organism on quinoline, 2-hydroxyquinoline accumulated as the intermediate; 8-hydroxycoumarin accumulated as the major metabolite on
O P Shukla
Microbios, 59(238), 47-63 (1989-01-01)
A Gram-negative, oxidase positive, polar flagellated rod, characterised as Pseudomonas stutzeri, has been isolated from sewage by enrichment culture on quinoline. The organism utilizes quinoline as the sole source of carbon, nitrogen and energy, and liberates UV absorbing and phenolic
Two polymorphs of 8-hydroxycarbostyril: X-ray crystallography, solid-state NMR and DFT calculations.
Nieto CI, et al.
Journal of Molecular Structure, 1008, 88-94 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service