SML4070
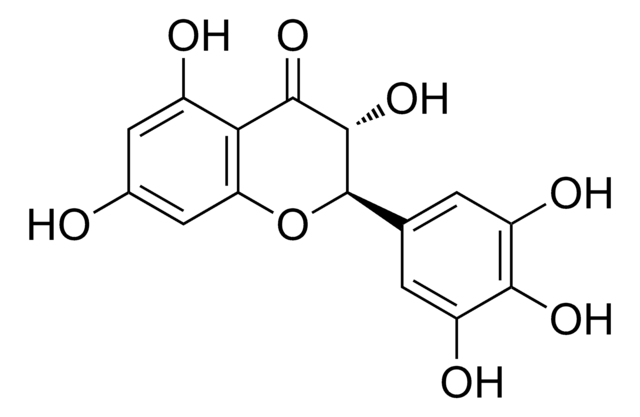
Dihydromyricetin

≥98% (HPLC)
Synonym(s):
(+)-Ampelopsin, (+)-Dihydromyricetin, (2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one, Ampelopsin, DHM
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H12O8
CAS Number:
Molecular Weight:
320.25
MDL number:
UNSPSC Code:
41116107
UNSPSC Code:
12352200
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
protect from light
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
-10 to -25°C
Biochem/physiol Actions
Antioxidant, anti-cancer flavanonol with anti-alcohol intoxication effects, significantly impacts metabolic regulation and cancer chemoprevention through pathways like AMPK and SPHK1/mTOR.Dihydromyricetin (DHM) is a potent flavonoid known for its antioxidative, anti-inflammatory, anticancer, and antimicrobial properties, and plays a key role in modulating metabolism with minimal side effects. It operates by either scavenging or generating reactive oxygen species (ROS) to selectively target cancer cells, while sparing normal cells. DHM acts through multiple pathways including AMPK, affecting autophagy and glucose transport. Additionally, Dihydromyricetin inhibits ferroptosis via the SPHK1/mTOR pathway, modulates the Prx2 cascade through Nrf2, and contributes to cancer prevention and diabetes treatment by altering redox balance, gene expression, and improving metabolism, also involving AMPK, PI3K/Akt, PPAR pathways, and microRNAs.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jie Fang et al.
Clinical and experimental pharmacology & physiology, 51(3), e13833-e13833 (2024-02-02)
Previous clinical reports have shown that capecitabine, an oral prodrug of 5-fluorouracil (5-Fu), can induce peripheral neuropathy, resulting in numbness, paresthesia and hypoesthesia. However, the mechanism through which capecitabine causes peripheral nerve injury remains unclear. Here, we demonstrate that systemic
Yi Shen et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(1), 390-401 (2012-01-06)
Alcohol use disorders (AUDs) constitute the most common form of substance abuse. The development of AUDs involves repeated alcohol use leading to tolerance, alcohol withdrawal syndrome, and physical and psychological dependence, with loss of ability to control excessive drinking. Currently
Zhenzhu Sun et al.
Biochemical pharmacology, 175, 113888-113888 (2020-03-01)
Doxorubicin (DOX) is a powerful anthracycline antineoplastic drug whose clinical application is limited by serious cardiotoxic side effects. Dihydromyricetin (DHM), a flavonoid compound extracted from the Japanese raisin tree (Hovenia dulcis), is cardioprotective in patients with heart failure; however, the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service