37520
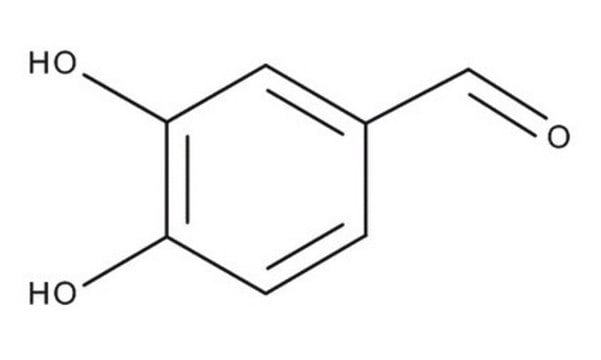
3,4-Dihydroxybenzaldehyde
purum, ≥97.0% (HPLC)
Synonym(s):
Protocatechualdehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
774381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (HPLC)
form
powder
mp
150-155 °C
150-157 °C (lit.)
SMILES string
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
InChI key
IBGBGRVKPALMCQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3,4-Dihydroxybenzaldehyde has been recognized as one of the antifungal compound extracted from the outer skin of green Cavendish bananas. It can be synthesized from catechol via Fries rearrangement.
3,4-Dihydroxybenzaldehyde is reported as bioactive compound which inhibits the H2O2-induced apoptosis of granulosa cells. Oxidation of 3,4-dihydroxybenzaldehyde on glassy carbon electrodes is reported to afford stable redox-active electropolymerized films containing a quinone moity.
Application
3,4-Dihydroxybenzaldehyde (Protocatechualdehyde) may be employed as starting reagent for the synthesis of 4-vinylbenzocrown ether.
3,4-Dihydroxybenzaldehyde may be used for the surface modification of nanocrystalline TiO2 particles. Electrodeposited layer of 3,4-dihydroxybenzaldehyde may be used as effective redox mediator during oxidation of NADH at graphene. It may be used in the preparation of new diSchiff base ligands, which forms di-, tri- and tetranuclear Co(II) and Cu(II) complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ahlam Jameel Abdulghani et al.
Bioinorganic chemistry and applications, 2013, 219356-219356 (2014-01-24)
A series of new di-, tri-, and tetranuclear Co(II) and Cu(II) complexes of three new diSchiff base ligands were synthesized by two different methods. The first method involved the synthesis of the three ligands from condensation reaction of 3,4-dihydroxybenzaldehyde (L'H2)
Ryohei Kono et al.
Acta histochemica et cytochemica, 47(3), 103-112 (2014-10-17)
Granulosa cells form ovarian follicles and play important roles in the growth and maturation of oocytes. The protection of granulosa cells from cellular injury caused by oxidative stress is an effective therapy for female infertility. We here investigated an effective
Ting Xie et al.
PeerJ, 7, e7690-e7690 (2019-10-03)
Lecanicillium lecanii is an entomopathogenic fungi, which was isolated from insects suffering from disease. Now, it is an effective bio-control resource that can control agricultural pests such as whitefly and aphids. There are many studies on the control of various
3, 4-dihydroxybenzaldehyde, a fungistatic substance from green Cavendish bananas.
Mulvena D, et al.
Phytochemistry, 8(2), 393-395 (1969)
Synthesis of 4'-vinylbenzocrown ethers.
Smid J, et al.
Organic Prep. and Proc. Int., 8(4), 193-196 (1976)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service