HCP2MAG62KPX23BK
MILLIPLEX® Human Cytokine/Chemokine Magnetic Bead Panel II - Premixed 23 Plex - Space Saver (Bulk) Packaging
Simultaneously analyze multiple cytokine and chemokine biomarkers with Bead-Based Multiplex Assays using the Luminex technology, in human serum, plasma and cell culture samples.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12161503
eCl@ss:
32161000
NACRES:
NA.47
Recommended Products
Quality Level
species reactivity
human
manufacturer/tradename
Milliplex®
assay range
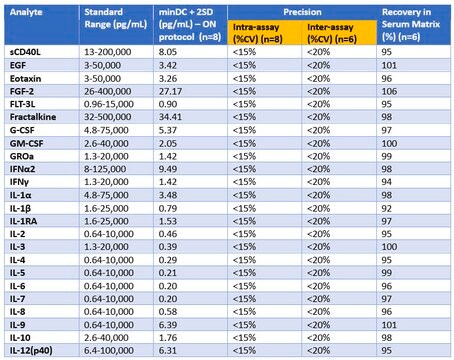
sensitivity: 0.4-55.8 pg/mL
standard curve range: 1.0-100,000 pg/mL
technique(s)
multiplexing: suitable
compatibility
configured for Premixed
detection method
fluorometric (Luminex xMAP)
shipped in
wet ice
General description
“Cytokine” is a general term used for a diverse group of soluble proteins and peptides which act as regulators under both normal and pathological conditions to modulate the functional activities of individual cells and tissues. These proteins also mediate direct interactions between cells and regulate processes taking place in the extracellular environment. The cytokine group of proteins includes lymphokines, interferons, colony stimulating factors and chemokines. Cytokine and chemokine research plays a significant role in achieving a deeper understanding of the immune system and its multi-faceted response to most antigens, as well as disease states such as inflammatory disease, allergic reactions, irritable bowel disease (IBD), sepsis, and cancer.
The MILLIPLEX® Human Cytokine / Chemokine Panel II enables you to focus on the therapeutic potential of cytokines as well as the modulation of cytokine expression.
The Luminex® xMAP® platform uses a magnetic bead immunoassay format for ideal speed and sensitivity to quantitate multiple analytes simultaneously, dramatically improving productivity while conserving valuable sample volume.
Panel Type: Cytokines/Chemokines
The MILLIPLEX® Human Cytokine / Chemokine Panel II enables you to focus on the therapeutic potential of cytokines as well as the modulation of cytokine expression.
The Luminex® xMAP® platform uses a magnetic bead immunoassay format for ideal speed and sensitivity to quantitate multiple analytes simultaneously, dramatically improving productivity while conserving valuable sample volume.
Panel Type: Cytokines/Chemokines
Specificity
Cross Reactivty
There was no or negligible cross-reactivity between the antibodies and any of the other analytes in this panel.
There was no or negligible cross-reactivity between the antibodies and any of the other analytes in this panel.
UPDATE >>
Cross-reactivity between the antibodies and any of the other analytes in this panel is non-detectable or negligible.
Cross-reactivity between the antibodies and any of the other analytes in this panel is non-detectable or negligible.
Application
- Analytes: 6Ckine, BCA-1, CTACK, ENA-78, Eotaxin-2, Eotaxin-3, I-309, IL-16, IL-20, IL-21, IL-23, IL-28A, IL-33, LIF, MCP-2, MCP-4, MIP-1δ, SCF, SDF-1A+β, TARC, TPO, TRAIL, TSLP
- Recommended Sample type: Serum, plasma, cell culture supernatant
- Recommended Sample dilution: neat
- Assay Run Time: Overnight or two-hour primary incubation. For best results, an overnight incubation is recommended
- Research Category: Inflammation & Immunology
- Research Subcategory: Obesity, Metabolic Disorders, Inflammation & Autoimmune Mechanisms
Packaging
96-well plate
Storage and Stability
Recommended storage for kit components is 2 - 8°C.
Other Notes
Please contact Technical Service for linearity of dilution.
Legal Information
Luminex is a registered trademark of Luminex Corp
MILLIPLEX is a registered trademark of Merck KGaA, Darmstadt, Germany
xMAP is a registered trademark of Luminex Corp
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Peter R Farrell et al.
The Journal of pediatrics (2021-07-18)
To utilize a Luminex platform to examine multiple cytokines simultaneously as well as clinical laboratory testing in order to identify markers that predict acute pancreatitis (AP) severity in the pediatric population on admission. Patients (<19 years) prospectively enrolled over a
Holden T Maecker et al.
Investigative radiology, 56(6), 374-384 (2021-01-16)
The aim of this study was to determine the following in patients who have undergone magnetic resonance imaging with gadolinium-based contrast agents (GBCAs) and meet the proposed diagnostic criteria for gadolinium deposition disease (GDD): (1) the effectiveness of chelation therapy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service