The stability of this material at -80°C has not been determined. In solid form, compounds are generally less sensitive to temperature, particularly for relatively short periods of time. It is likely that the quality has not been impacted. However this cannot be guaranteed. It would be up to the end user to determine the suitability for use.
584222
Tetrahydrouridine
Potent competitive inhibitor of cytidine deaminase. Also available as a 100 mM solution in H2O.
Synonym(s):
Tetrahydrouridine
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
Assay
≥90% (TLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
color
white to off-white
solubility
water: 200 mg/mL
shipped in
wet ice
storage temp.
−20°C
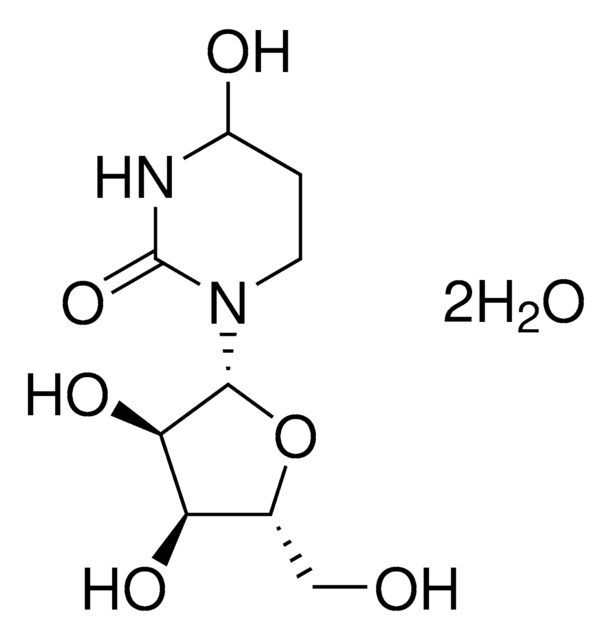
SMILES string
N2(CCC(NC2=O)O)[C@@H]1O[C@@H]([C@H]([C@H]1O)O)CO
InChI
1S/C9H16N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h4-8,12-15H,1-3H2,(H,10,16)/t4-,5?,6-,7-,8-/m1/s1
InChI key
UCKYOOZPSJFJIZ-XVKVHKPRSA-N
Related Categories
General description
Application
- Oncotherapy resistance explained by Darwinian and Lamarckian models.: This research explores the mechanisms of oncotherapy resistance, combining Darwinian and Lamarckian models to provide a comprehensive understanding. The study highlights the role of Tetrahydrouridine in modulating therapy resistance pathways in cancer treatment (Saunthararajah et al., 2024).
- Neuroendocrine lineage commitment of small cell lung cancers can be leveraged into p53-independent non-cytotoxic therapy.: This study investigates the potential of using Tetrahydrouridine in non-cytotoxic therapies for small cell lung cancers, focusing on neuroendocrine lineage commitment and its implications for treatment efficacy (Biswas et al., 2023).
- In Vitro Interaction of Tetrahydrouridine with Key Human Nucleoside Transporters.: This study explores how Tetrahydrouridine interacts with human nucleoside transporters, shedding light on its mechanisms of action and potential as a therapeutic agent in biochemistry and pharmacology (Säll et al., 2023).
- Mycoplasma infection of cancer cells enhances anti-tumor effect of oxidized methylcytidines.: This research investigates how mycoplasma infection enhances the anti-tumor effects of oxidized methylcytidines, with Tetrahydrouridine playing a crucial role in the observed therapeutic outcomes (Pang et al., 2023).
Packaging
Warning
Reconstitution
Other Notes
Bouffard, D.Y., et al. 1993. Biochem Pharmacol.45, 1857.
Laliberte, J., et al. 1992. Cancer Chemotherap. Pharmacol.30, 7.
Riva, C., et al. 1992. Chemotherapy38, 358.
Yusa, K., et al. 1992. J. Biol. Chem. 267, 16848.
Hanze, A.R. 1967. J. Am. Chem. Soc.89, 6720.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
-
Dear partener, Kindly advice if THU 10 mg vials sored at -80 for 3 days can be used. Thank you,
1 answer-
Helpful?
-
-
Hi, may I ask if the THU (Cat number: 18771-50-1) is stable if stored at -4 oC and 10 oC? There is temperature excursion of the freezer in lab, and we would like to check what is the highest tolerable storage Temp which this reagent stored
1 answer-
The recommended storage temperature for this material is −20°C. The product is shipped on ice to maintain the quality of the compound. In general, products of this nature are considered stable within the range of −10 to 25°C. The end user would have to evaluate the integrity of the product for excursions beyond this range.
Helpful?
-
-
Does this product have an expiry date?
1 answer-
This product does not contain an expiration date. It contains a recommended retest date of 10 years after the quality release date. A recommended retest date is the period of time during which the product is expected to remain within established stability specifications, provided that it has been stored under defined conditions. After the Retest Date, product samples should be examined to ensure that the product is still in compliance with the established specifications. For more information, you may access the "Product Dating Information" document under "ADDITIONAL USEFUL DOCUMENTS ABOUT OUR PRODUCTS" at the bottom of the Quality Services page with this link: https://www.sigmaaldrich.com/US/en/life-science/quality-and-regulatory-management/quality-services
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service