219362
Cathepsin B, Human Liver
Cathepsin B, Human Liver, CAS 9047-22-7, is a purified native cathepsin B from human liver, purified by affinity chromatography. Upregulated in many types of tumors.
Synonym(s):
Cathepsin B, Human Liver, cat B, cysteine cathepsin
About This Item
Recommended Products
biological source
human liver
Quality Level
Assay
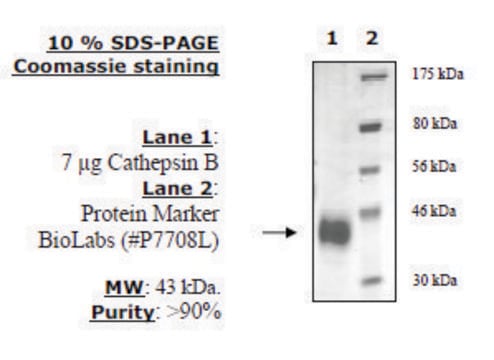
≥95% (SDS-PAGE)
form
liquid
specific activity
≥10 units/mg protein
purified by
affinity chromatography
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
avoid repeated freeze/thaw cycles
technique(s)
activity assay: suitable
suitability
suitable for molecular biology
application(s)
life science and biopharma
shipped in
wet ice
storage temp.
−70°C
Gene Information
human ... CTSB(1508)
General description
Native cathepsin B from human liver, purified by affinity chromatography and HPLC. The most investigated enzyme of all lysosomal cysteine proteases. Cathepsin B belongs to the papain-like family of cysteine proteases and is produced as a preproenzyme. It is a bilobal protein, and its catalytic site is situated at the interface between the two lobes.
Application
- Diagnostics: as a potent and independent prognostic marker for endometrial cancer, pancreatic adenocarcinoma, and inflammatory disease.
- Drug development: during the neovascularization process and as a potent therapeutic target for various pathologies, cancer progression, and osteoarthritis in humans.
- Pharmacology: for increasing the therapeutic index of doxorubicin by incorporating the cathepsin B cleavable spacer Phe-Lys-4-aminobenzyloxycarbonyl into an albumin-binding prodrug.
- Molecular biology: in cathepsin B activity assay.
Biochem/physiol Actions
Warning
Unit Definition
Physical form
Preparation Note
Reconstitution
Other Notes
Kostoulas, G., et al. 1999. FEBS Lett.455, 286.
Strojnik, T., et al. 1999. Clin. Cancer Res.5, 559.
Maquire, T.M., et al. 1998. Int. J. Biol. Markers13, 139.
Berquim, I.M., and Sloane, B.F. 1996. Adv. Exp. Med. Biol.389, 281.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service