All Photos(1)
About This Item
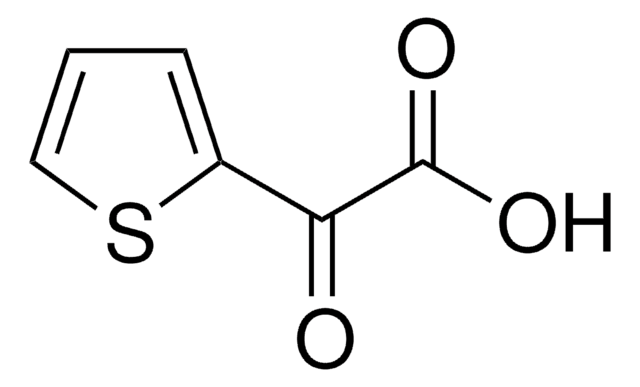
Empirical Formula (Hill Notation):
C6H10O3
CAS Number:
Molecular Weight:
130.14
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
Recommended Products
form
liquid
SMILES string
CC(C)(C)C(=O)C(O)=O
InChI
1S/C6H10O3/c1-6(2,3)4(7)5(8)9/h1-3H3,(H,8,9)
InChI key
IAWVHZJZHDSEOC-UHFFFAOYSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wei Jiang et al.
Journal of biotechnology, 292, 50-56 (2019-01-29)
A bioinspired strategy for the synthesis of supramolecular and biocatalytical materials was developed base on protein-protein supramolecular interaction and genetic engineering. Formate dehydrogenase (FDH) and its functional fragments were separately fused to form a multi-function domain. The fusion proteins and
Langxing Liao et al.
Microbial cell factories, 20(1), 3-3 (2021-01-08)
Biosynthesis of L-tert-leucine (L-tle), a significant pharmaceutical intermediate, by a cofactor regeneration system friendly and efficiently is a worthful goal all the time. The cofactor regeneration system of leucine dehydrogenase (LeuDH) and glucose dehydrogenase (GDH) has showed great coupling catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service