426091
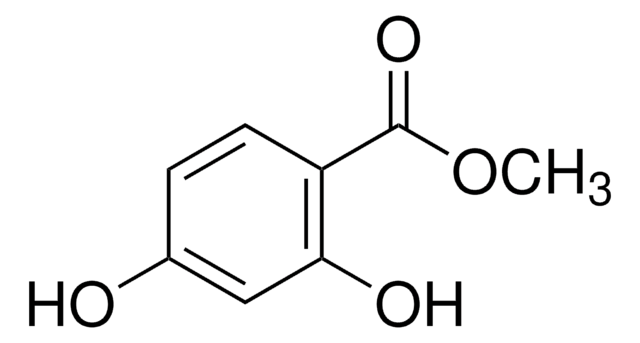
Methyl 2,5-dihydroxybenzoate
99%
Synonym(s):
Methyl gentisate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H3CO2CH3
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
86-88 °C (lit.)
SMILES string
COC(=O)c1cc(O)ccc1O
InChI
1S/C8H8O4/c1-12-8(11)6-4-5(9)2-3-7(6)10/h2-4,9-10H,1H3
InChI key
XGDPKUKRQHHZTH-UHFFFAOYSA-N
Related Categories
General description
Methyl 2,5-dihydroxybenzoate (methyl gentisate) is an alkyl ester of gentisic acid. It is reported to show less cytotoxic and mutagenic activity than hydroquinone with a potential to inhibit melanogenesis. It has been synthesized from 2,5-dihydroxybenzoic acid. The crystal structure of the molecule was found to be planar.
Application
Methyl 2,5-dihydroxybenzoate (methyl gentisate) may be used as a starting material in the synthesis of euonyminol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E V Curto et al.
Biochemical pharmacology, 57(6), 663-672 (1999-02-26)
To discover safe and effective topical skin-lightening agents, we have evaluated alkyl esters of the natural product gentisic acid (GA), which is related to our lead compound methyl gentisate (MG), and four putative tyrosinase inhibitors, utilizing mammalian melanocyte cell cultures
Methyl 2, 5-dihydroxybenzoate.
Brown CL, et al.
Acta Crystallographica Section E, Structure Reports Online, 59(5), 630-631 (2003)
Total Synthesis of (.+-.)-Euonyminol, the Sesquiterpenoid Nucleus of Cathedulin K-19, via an Epoxide Cascade Cyclization.
White JD, et al.
Journal of the American Chemical Society, 117(38), 9780-9781 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service