All Photos(1)
About This Item
Linear Formula:
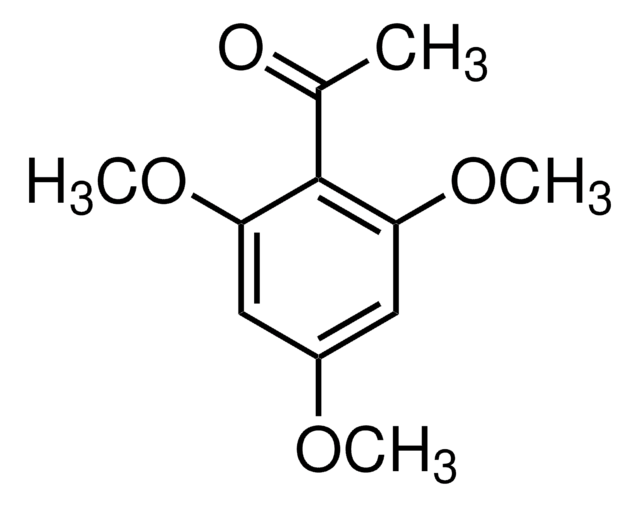
(CH3O)2C6H3COCH3
CAS Number:
Molecular Weight:
180.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
135-136 °C/2 mmHg (lit.)
mp
68-70 °C (lit.)
functional group
ketone
SMILES string
COc1cccc(OC)c1C(C)=O
InChI
1S/C10H12O3/c1-7(11)10-8(12-2)5-4-6-9(10)13-3/h4-6H,1-3H3
InChI key
XEUGKOFTNAYMMX-UHFFFAOYSA-N
General description
2,6-Dihydroxyacetophenone, its mono- and di-methyl ethers are inhibitors of hepatic mixed function oxidases. Metabolism of 2′,6′-dimethoxyacetophenone was studied.
Application
2′,6′-Dimethoxyacetophenone was used in preparation of 4-fluororesorcinol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Bobik et al.
Xenobiotica; the fate of foreign compounds in biological systems, 5(2), 65-72 (1975-02-01)
1. 2,6-Dihydroxyacetophenone, its mono- and di-methyl ethers are inhibitors of hepatic mixed function oxidases. The dimethyl ether is a competitive inhibitor of aminopyrine demethylase with the others displaying mixed kinetics. The metabolism of all three ketones has been studied. 2.
Facile preparations of 4-fluororesorcinol.
Belanger PC, et al.
Canadian Journal of Chemistry, 66(6), 1479-1482 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service