270962
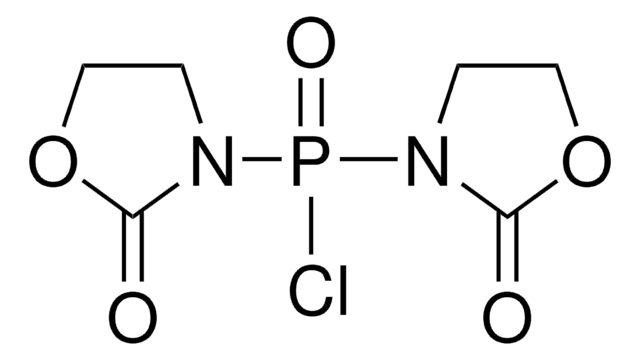
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride
97%
Synonym(s):
BOP-Cl, Phosphoric acid bis(2-oxooxazolidide) chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8ClN2O5P
CAS Number:
Molecular Weight:
254.56
Beilstein:
3654596
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
191 °C (dec.) (lit.)
SMILES string
ClP(=O)(N1CCOC1=O)N2CCOC2=O
InChI
1S/C6H8ClN2O5P/c7-15(12,8-1-3-13-5(8)10)9-2-4-14-6(9)11/h1-4H2
InChI key
KLDLRDSRCMJKGM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent for activating carboxyl groups and coupling peptides.
Other Notes
This product has been replaced by 15140-ALDRICH | Bis(2-oxo-3-oxazolidinyl)phosphinic chloride ≥97.0% (AT)
replaced by
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nonionic surfactants regioselective synthesis of fatty acid esters of alpha- and beta-glucopyranose.
D De Luca et al.
Lipids, 32(5), 559-563 (1997-05-01)
Lipophilic esters of saccharides belong to the family of nonionic surfactants widely employed in pharmaceutical and cosmetics formulations. A very simple method is presented whereby 6-O-esters of alpha- and beta-glucose can be prepared and isolated. Good results have been obtained
H T Le et al.
Bioorganic & medicinal chemistry, 4(12), 2201-2209 (1996-12-01)
BOP-Cl was found to be an efficient coupling reagent for the introduction of thiopeptide bonds on imino acid residues (Pro, Sar). Boc-amino monothioacids were coupled at moderate temperature (0 degree C-RT) with fair yields and with retained optical purity. The
T Miyazawa et al.
International journal of peptide and protein research, 40(1), 49-53 (1992-07-01)
In segment couplings by the mixed anhydride method using isobutyloxycarbonyl chloride, the use of copper(II) chloride as an additive suppressed racemization completely in the same manner as in the carbodiimide method reported previously. This was confirmed by employing a number
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service