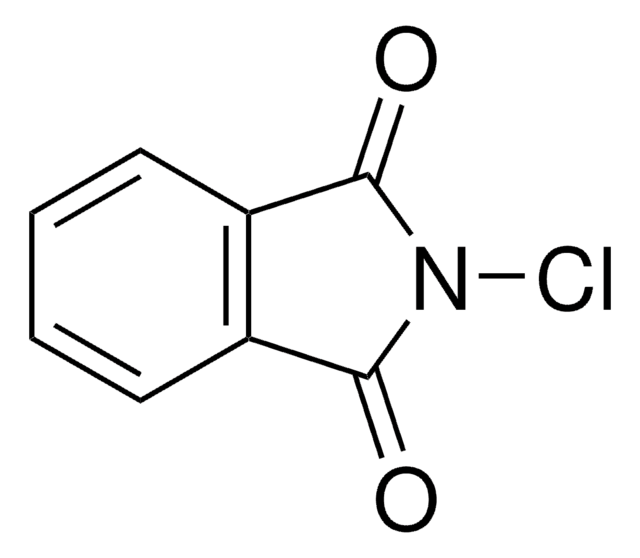
220051
N-Iodosuccinimide
95%
Synonym(s):
1-iodo-pyrrolidine-2,5-dione, 1-iodoazolidine-2,5-dione, NIS, succiniodimide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4INO2
CAS Number:
Molecular Weight:
224.98
Beilstein:
113917
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
form:
powder
Assay:
95%
Recommended Products
Quality Level
Assay
95%
form
powder
mp
202-206 °C (lit.)
functional group
imide
storage temp.
2-8°C
SMILES string
IN1C(=O)CCC1=O
InChI
1S/C4H4INO2/c5-6-3(7)1-2-4(6)8/h1-2H2
InChI key
LQZMLBORDGWNPD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Highly substituted iodobenzenes prepared via an efficient 2-step process from 1,6-diynes. Used with TFA to chemoselectively hydrolyze thioglycosides to 1-hydroxyglycosides. Synthesis of vinyl sulfones from olefins and benzenesulfinic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Canadian Journal of Chemistry, 84, 66-66 (2006)
Yoshihiko Yamamoto et al.
Journal of the American Chemical Society, 128(25), 8336-8340 (2006-06-22)
Highly substituted iodobenzenes were efficiently and regioselectively synthesized from readily available 1,6-diynes via two-step process consisting of silver-catalyzed Csp-H iodination and subsequent ruthenium-catalyzed [2 + 2 + 2] cycloaddition of resultant iododiynes. Some of the obtained iodobenzenes were subjected to
Taichi Kano et al.
Journal of the American Chemical Society, 130(12), 3728-3729 (2008-03-07)
A direct asymmetric iodination reaction of aldehydes with NIS was found to be catalyzed by the novel axially chiral bifunctional amino alcohol (S)-1d. This method represents the rare example of the catalytic and highly enantioselective synthesis of optically active alpha-iodoaldehydes.
Yuanxun Zhu et al.
Organic letters, 13(5), 1024-1027 (2011-01-28)
An efficient and straightforward strategy for the synthesis of N-(2-haloinden-1-yl)arenesulfonamides from propargylic alcohols and sulfonamides is described. Allenesulfonamide is postulated to be the key intermediate for this tandem transformation.
Abby J Isaacs et al.
The Journal of thoracic and cardiovascular surgery, 149(5), 1262-1269 (2015-03-21)
Substantial controversy surrounds the choice between a mechanical versus bioprosthetic prosthesis for aortic valve replacement (AVR), based on age. This study aims to investigate national trends and in-hospital outcomes of the 2 prosthesis choices. All patients aged >18 years in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)