All Photos(1)
About This Item
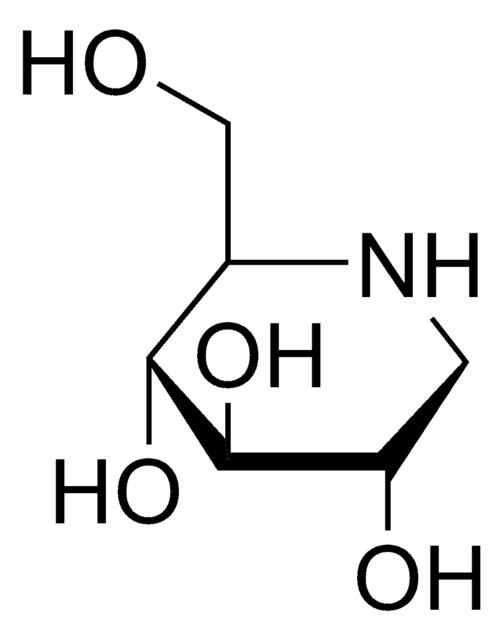
Empirical Formula (Hill Notation):
C7H15NO5
CAS Number:
Molecular Weight:
193.20
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98.0% (TLC)
SMILES string
OC[C@H]1N[C@H](CO)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6+,7+/m1/s1
InChI key
CLVUFWXGNIFGNC-OVHBTUCOSA-N
Application
α-Homonojirimycin (HMJ) is used as an inhibitor of several carbohydrate degrading enzymes including α-glucosidases, glycoprotein processing enzyme glucosidase II and maltase.
Biochem/physiol Actions
α-Homonojirimycin is a potent inhibitor of a range of α-glucosidases, as well as an inhibitor of the glycoprotein processing enzyme glucosidase II.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K Ikeda et al.
Carbohydrate research, 323(1-4), 73-80 (2000-04-27)
2,6-Dideoxy-7-O-(beta-D-glucopyranosyl) 2,6-imino-D-glycero-L-gulo- heptitol (7-O-beta-D-glucopyranosyl-alpha-homonojirimycin, 1) was isolated from the 50% methanol extract of the whole plant of Lobelia sessilifolia (Campanulaceae), which was found to potently inhibit rice alpha-glucosidase. Adenophorae radix, roots of Adenophora spp. (Campanulaceae), yielded new homonojirimycin derivatives, adenophorine
Gabriel M J Lenagh-Snow et al.
Organic letters, 14(8), 2050-2053 (2012-04-05)
Although there are 32 6-azidoheptitols, there are only 16 homonojirimycin (HNJ) stereoisomers. Two epimeric azidoalditols derived from d-mannose allow the synthesis in water of eight stereoisomers of HNJ.
O R Martin et al.
Bioorganic & medicinal chemistry letters, 9(21), 3171-3174 (1999-11-24)
The structure of a homonojirimycin isomer isolated from Aglaonema treublii and originally proposed as alpha-3,4-di-epi-homonojirimycin was revised to alpha-4-epi-homonojirimycin 3 ("alpha-homoallonojirimycin") on the basis of NMR analysis and synthetic studies. Its activity as a glycosidase inhibitor is compared to that
Shankar D Markad et al.
Bioorganic & medicinal chemistry, 14(16), 5535-5539 (2006-05-10)
Conjugate addition of n-butyl amine to d-glucose derived alpha,beta-unsaturated ester 4 afforded beta-amino esters 5a,b that on reduction of ester group, 1,2-acetonide deprotection, and reductive amination led to the formation of corresponding N-butyl 1-deoxy-D-gluco-homonojirimycin 2c and N-butyl 1-deoxy-L-ido-homonojirimycin 2d which
Chinami Kuriyama et al.
Bioorganic & medicinal chemistry, 16(15), 7330-7336 (2008-07-04)
We investigated in vitro inhibition of mammalian carbohydrate-degrading enzymes by six-membered sugar mimics and their evaluation in cell cultures. 1-Deoxynojirimycin (DNJ) showed no significant inhibition toward glycogen phosphorylase (GP) but was a potent inhibitor of another glycogen-degrading enzyme, amylo-1,6-glucosidase (1,6-GL)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service