163031
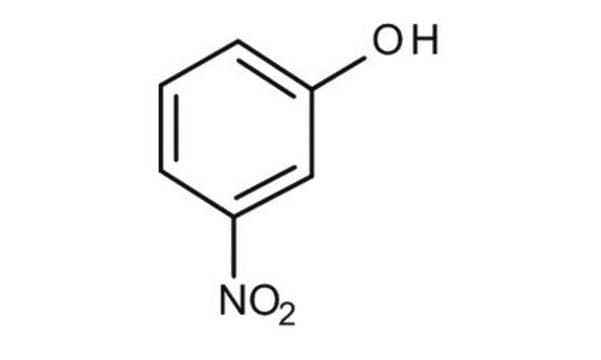
3-Nitrophenol
ReagentPlus®, 99%
Synonym(s):
3-NP, m-NP, m-Nitrophenol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4OH
CAS Number:
Molecular Weight:
139.11
Beilstein:
1907946
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
194 °C/70 mmHg (lit.)
mp
96-98 °C (lit.)
SMILES string
Oc1cccc(c1)[N+]([O-])=O
InChI
1S/C6H5NO3/c8-6-3-1-2-5(4-6)7(9)10/h1-4,8H
InChI key
RTZZCYNQPHTPPL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Nitrophenol (m-nitrophenol) is a nitroaromatic compound. It can be prepared from 3-nitroaniline, via diazotization reaction.
3-Nitrophenol is one of the isomers of mononitrophenol and is mainly used as an intermediate to prepare dyes, pigments, lumber preservatives, photographic chemicals and pesticides. Some of the methods for its degradation are biotransformation, photocatalytic degradation and photooxidation.
Application
3-Nitrophenol may be used in the preparation of 3-aminophenol. It may be employed as a weak acid in capillary isoelectric focusing (cIEF) method.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways.
Haigler BE and Spain JC.
Applied and Environmental Microbiology, 57(11), 3156-3162 (1991)
Thierry Vincent et al.
Environmental science & technology, 38(15), 4233-4240 (2004-09-09)
Hollow chitosan fibers were reacted with chloropalladate solutions and subsequently reduced by hydrogen produced in situ by reaction of sulfuric acid with zinc powder in order to manufacture palladium supported on catalytic hollow chitosan fibers (C2HF-Pd). This catalytic support was
Z Ioannou et al.
Journal of hazardous materials, 171(1-3), 954-964 (2009-07-25)
Carbonaceous adsorbents prepared from olive stones biomass and novolac resin, as well as a commercial activated carbon for comparison reasons, have been examined for the removal of phenol and 3-nitrophenol from aqueous solutions. All carbonaceous adsorbents have been characterized by
J S Zhao et al.
Applied and environmental microbiology, 67(3), 1388-1391 (2001-03-07)
The 3-nitrophenol-induced enzyme system in cells of Pseudomonas putida 2NP8 manifested a wide substrate range in transforming nitroaromatic compounds through to ammonia production. All of the 30 mono- or dinitroaromatic substrates except 4-nitrophenol, 2,4-dinitrophenol, 2,4,6-trinitrophenol, 3-nitroaniline, 2-nitrobenzoic acid, and 2-nitrofuran
Epoxy-based oligomer bearing naphthalene units: fluorescent sensor for 4-nitrophenol.
Ghosh S.
Tetrahedron Letters, 56(48), 6738-6741 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service