AG210
Prion Protein, recombinant
Synonym(s):
PrP, CD230
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.26
Recommended Products
biological source
bovine
Quality Level
Assay
>95% (total protein)
form
liquid
manufacturer/tradename
Chemicon®
technique(s)
cell based assay: suitable
NCBI accession no.
UniProt accession no.
shipped in
dry ice
Gene Information
bovine (calf) ... PRNP(281427)
General description
Histidine-tagged full-length mature part of bovine PrP (25-244) is expressed in E. Coli BL21, solubilized from inclusion bodies in 6 M guanidine-HCl, and purified by Ni(II)-nitriloacetate agarose chromatography followed by reversed-phase HPLC (C4 column)
Prion diseases or transmissible spongiform encephalopathies are neurodegenerative diseases that affect both humans and animals (Prusiner 1998). All prion diseases share the same molecular pathogenic mechanism that involves conversion of normal cellular prion protein (PrPc) into a form that is insoluble in non ionic detergent and partially resistant to proteases (PrPSc) (Pan et al. 1993). Both PrPSc and PrPc are encoded within a single exon of a chromosomal gene as a protein of ~ 250 amino acids (Basler et al. 1986). Many mammalian PrPs have a 22 amino acid N-terminal signal sequence (Hope et al. 1986; Turk et al. 1988) and 23 amino acid C-terminal signal sequence encoding for attachment of a glycosylphosphatidylinositol anchor (Stahl et al. 1987, 1990). The mature protein of 209 amino acids contains one disulfide bond (Turk et al. 1988) and has two sites of asparagine-linked glycosylation (Endo et al. 1989; Oesch et al. 1995).
Application
Antigen in standard immunochemical detection of BSE.
Optimal working dilution must be determined by the end user.
Optimal working dilution must be determined by the end user.
Research Category
Neuroscience
Neuroscience
Research Sub Category
Neurodegenerative Diseases
Neurodegenerative Diseases
Physical form
Liquid in 10 mM sodium acetate buffer, pH 4.0, containing 0.01% sodium azide.
Storage and Stability
Maintain at -20°C in undiluted aliquots for up to 6 months after date of receipt. Avoid repeated freeze/thaw cycles.
Analysis Note
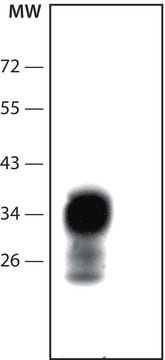
The PrPc appears as a single band of about 27 kD by SDS-PAGE
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins.
Pan, K M, et al.
Proceedings of the National Academy of Sciences of the USA, 90, 10962-10966 (1993)
Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein.
Endo, T, et al.
Biochemistry, 28, 8380-8388 (1989)
Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene.
Basler, K, et al.
Cell, 46, 417-428 (1986)
Purification and properties of the cellular and scrapie hamster prion proteins.
Turk, E, et al.
European Journal of Biochemistry, 176, 21-30 (1988)
Identification of glycoinositol phospholipid linked and truncated forms of the scrapie prion protein.
Stahl, N, et al.
Biochemistry, 29, 8879-8884 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service