912034
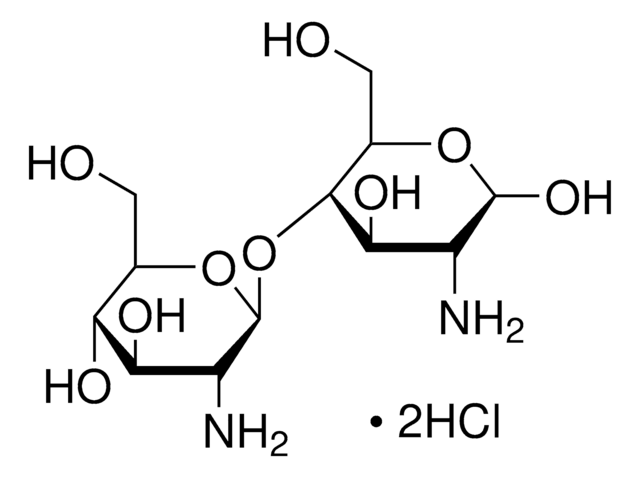
Trimethyl chitosan
high molecular weight, degree of quaternization 30-70%
Synonym(s):
N,N,N-Trimethyl chitosan, Chitosan trimethyl, Mucoadhesive polymers, TMC Chitosan
About This Item
Recommended Products
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
The quaternization of the primary amine increases the water solubility of chitosan and keeps chitosan soluble over a wide pH range. Among all the quaternized chitosans, N, N, N-trimethyl chitosan chloride (TMC) is the most widely applied in biomedical applications.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Professor Robert K. Prud’homme introduces flash nanoprecipitation (FNP) for nanoparticle fabrication, which is a scalable, rapid mixing process for nanoparticle formulations.
Microfluidic assembly improves polyamine nanoencapsulation of nucleic acids, overcoming challenges like polydispersity and poor reproducibility.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service