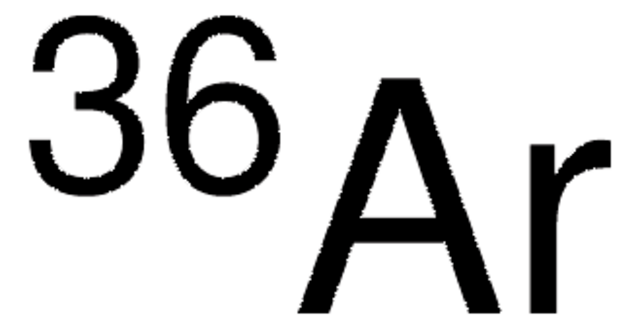
768952
Argon
Messer® CANGas, 99.999%
Synonym(s):
Argon gas
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
Ar
CAS Number:
Molecular Weight:
39.95
EC Number:
MDL number:
UNSPSC Code:
12142004
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1.38 (21 °C, vs air)
Quality Level
description
Filling mass: 20g
Filling volume: 12L
Assay
99.999%
form
gas
bp
-185.7 °C (lit.)
mp
-189.2 °C (lit.)
SMILES string
[Ar]
InChI
1S/Ar
InChI key
XKRFYHLGVUSROY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Argon is a rare gas that is colourless, odourless, and heavier than air. Applications for argon include use as a shielding gas for special welding problems and sensitive materials (titanium, niobium, tungsten, etc.), use as a filling gas for windows and lamps, use in spark erosion spectrometry and plasma processes. Gas is easy to handle in a small, light pressure can, while the disposable container allows for aluminium recycling when finished.
Recommended products
Z742374: CANgas pressure regulator
Z742379: CANgas plastic hose spout
Z742378: CANgas outer adaptor
Z742377: CANgas inner adaptor
Z742375: CANgas dosing valve
Z742379: CANgas plastic hose spout
Z742378: CANgas outer adaptor
Z742377: CANgas inner adaptor
Z742375: CANgas dosing valve
Legal Information
Messer is a registered trademark of Messer Group GmbH
accessory
regulator
Product No.
Description
Pricing
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Press. Gas Compr. Gas
Storage Class Code
2A - Gases
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Justin P Wiens et al.
The Journal of chemical physics, 145(24), 244312-244312 (2017-01-05)
Dissociative recombination of electrons with HCl
Yahya Safari et al.
Journal of the science of food and agriculture, 98(8), 2915-2924 (2017-11-22)
Persistent sample circulation microextraction (PSCME) combined with graphite furnace atomic absorption spectrometry (GFAAS) was developed as a high pre-concentration technique for the determination of heavy metals in fish species. In this method, a few microliters of organic solvent (40.0 µL carbon
Xiaotong Jiang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 191, 155-164 (2017-10-14)
The docking sites of hydrogen bonds in complexes formed between 2,2,2-trifluoroethanol (TFE), furan (Fu), and 2-methyl furan (MF) have been investigated. Using density functional theory (DFT) calculations, gas phase and matrix isolation FTIR spectroscopies, the strengths of OH⋯O and OH⋯π
Jonas Häusler et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(11), 2583-2590 (2016-12-04)
The first gallium-containing nitridosilicate CaGaSiN
T Urbańczyk et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 196, 58-66 (2018-02-13)
Revisited study of the E3Σ1+ (63S1)←A3Π0+(53P1) transition in CdAr using both theoretical and experimental approach is presented. Systematic detection of the E3Σ1+in,υ'←A3Π0+,υ″=6 transition frequencies with higher accuracy and spectrally narrower laser extended and improved analysis and simulation of the LIF
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service