All Photos(1)
About This Item
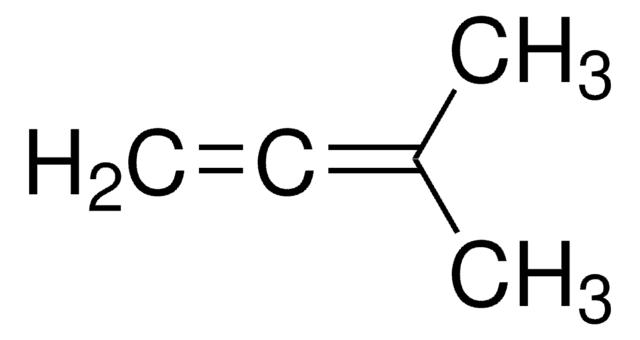
Linear Formula:
CH2=C=CHCH3
CAS Number:
Molecular Weight:
54.09
Beilstein:
1730808
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0%
form
gas
bp
10.8 °C (lit.)
SMILES string
CC=C=C
InChI
1S/C4H6/c1-3-4-2/h4H,1H2,2H3
InChI key
QNRMTGGDHLBXQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,2-Butadiene can be used as a reagent:
- To synthesize useful β-borylallylsilanes building blocks by reacting with various achiral silylboranes via Pd-catalyzed asymmetric silaboration reaction.
- In the enantioselective diboration reaction in the presence of palladium catalyst and Lewis base ligand.
- 3-methyl-4-methyleneisocoumarin via formation of ortho-thallated benzoic acid in the presence of thallium trifluoroacetate and palladium catalyst.
Packaging
500ml high pressure aluminum cylinder equipped with a stainless steel valve according to DIN 477-1.
Other Notes
For 743704-250g-EU packaging: 500 mL of aluminium cylinder equipped with brass valve.
Recommended products
For 743704-250g-EU: outlet fitting: DIN 477-1 no. 1, Z742160 or Z742161 are the recommended regulators.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of isocoumarins via thallation-olefination of benzoic acids
Larock RC, et al.
Journal of the American Chemical Society, 106(18), 5274-5284 (1984)
Palladium-catalyzed enantioselective diboration of prochiral allenes
Pelz NF, et al.
Journal of the American Chemical Society, 126(50), 16328-16329 (2004)
Palladium-catalyzed asymmetric silaboration of allenes.
Ohmura T, et al.
Journal of the American Chemical Society, 128(42), 13682-13683 (2006)
Ralf I Kaiser et al.
The journal of physical chemistry. A, 118(32), 6181-6190 (2014-08-02)
The reactions of the 4-tolyl radical (C6H4CH3) and of the D7-4-tolyl radical (C6D4CD3) with 1,2-butadiene (C4H6) have been probed in crossed molecular beams under single collision conditions at a collision energy of about 54 kJ mol(-1) and studied theoretically using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service