715468
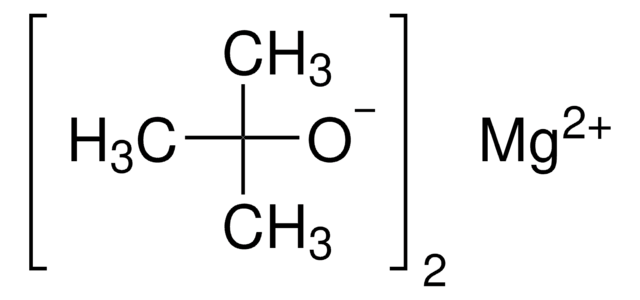
Barium tert-butoxide
85%
Synonym(s):
Barium di-t-butoxide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H18BaO2
CAS Number:
Molecular Weight:
283.55
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
85%
form
powder
mp
>200 °C
storage temp.
2-8°C
SMILES string
CC(C)(C)O[Ba]OC(C)(C)C
InChI
1S/2C4H9O.Ba/c2*1-4(2,3)5;/h2*1-3H3;/q2*-1;+2
InChI key
SLPLCLDJTNLWPW-UHFFFAOYSA-N
Related Categories
General description
Barium tert-butoxide is a Brønsted base catalyst that can be used as a precursor of various barium catalysts like barium aryloxide. It can also be used in the preparation of barium diphenylmethanide, applicable in the organometallic chemistry.
Application
Barium tert-butoxide can be used:
- As a catalyst along with various phenolic ligands in the aldol reactions of amides with aldehydes and ketones.
- To prepare yttrium and copper metal complexes by reacting with Y(thd)3 and Cu(thd)2, where “thd” is 2,2,6,6-tetramethylheptane-3,5-dione.
- As an initiator along with an alkyl lithium in the butadiene copolymerization reaction.
- In the iridium catalyzed allylic alkylation of non-stabilized enolates.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iridium-catalyzed diastereo-, enantio-, and regioselective allylic alkylation with prochiral enolates
Hethcox JC, et al.
ACS Catalysis, 6(9), 6207-6213 (2016)
Reactivity of mixed-metal BaCu and BaY β-diketonatoalkoxides with lewis bases: Molecular structures of [Ba2Cu (μ3, η2-OCHMeCH2NMe2) 2 (μ, η2-thd) 2 (η2-thd) 2 (PriOH2],[Cu3 (μ3-OBut) 2 (μ-OBut)(η2-thd) 3] and [Ba (η2-thd) 2 (TMEDA) 2]
Labrize F, et al.
Polyhedron, 15(4), 577-589 (1996)
Highly anti-Selective Catalytic Aldol Reactions of Amides with Aldehydes
Saito S and Kobayashi S
Journal of the American Chemical Society, 128(27), 8704-8705 (2006)
Barium tert-butoxide
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2009)
Accessible microstructures of polybutadiene by anionic polymerization
Forens A, et al.
Polymer, 153(4), 103-122 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service