440884
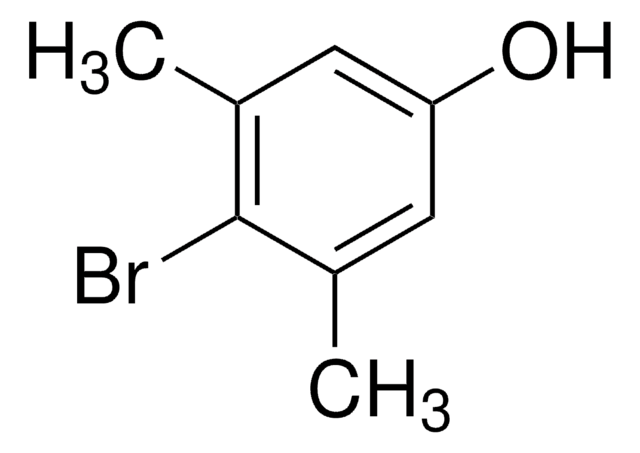
4-Bromo-3-methylphenol
98%
Synonym(s):
4-Bromo-m-cresol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H3(CH3)OH
CAS Number:
Molecular Weight:
187.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
142-145 °C/23 mmHg (lit.)
mp
59-61 °C (lit.)
SMILES string
Cc1cc(O)ccc1Br
InChI
1S/C7H7BrO/c1-5-4-6(9)2-3-7(5)8/h2-4,9H,1H3
InChI key
GPOQODYGMUTOQL-UHFFFAOYSA-N
General description
4-Bromo-3-methylphenol is a brominated phenol. It participates in the total synthesis of 3-methylcalix[4]arene.
Application
4-Bromo-3-methylphenol may be used in the synthesis of bromo[2-methyl-4-[2-(t-butyldimethylsilyloxy)ethyloxy]phenyl]bis(triphenylphopshine)nickel(II) (protected alcohol-functionalized initiator) and bromo-[2-methyl-4-[6-(t-butyldimethylsilyloxy)hexyloxy]phenyl] bis(triphenylphosphine) nickel.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Influence of the Presence and Length of an Alkyl Spacer on the Supramolecular Chirality of Block Copoly (thiophene) s.
Van den Bergh K, et al.
Macromolecules, 44(4), 728-735 (2011)
SOME MONO AND DIBROMO DERIVATIVES OF META-CRESOL.
Huston RC and Hutchinson JA.
Journal of the American Chemical Society, 54(4), 1504-1506 (1932)
End group-functionalization and synthesis of block-copolythiophenes by modified nickel initiators.
Smeets A, et al.
Macromolecules, 44(5), 6017-6025 (2011)
3-Methylcalix[4]arene: A New Versatile Precursor to Inherently Chiral Calix[4]arenes.
Dian-Kui Fu et al.
The Journal of organic chemistry, 61(2), 802-804 (1996-01-26)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service