377619
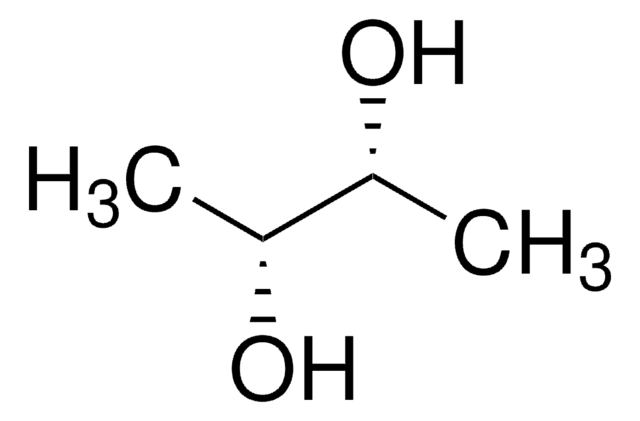
D-Threitol
99%
Synonym(s):
(2R,3R)-1,2,3,4-Butanetetrol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2[CH(OH)]2CH2OH
CAS Number:
Molecular Weight:
122.12
Beilstein:
1719752
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]20/D −14°, c = 2 in ethanol
mp
88-90 °C (lit.)
SMILES string
OC[C@@H](O)[C@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
UNXHWFMMPAWVPI-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Döss et al.
Physical review letters, 88(9), 095701-095701 (2002-02-28)
We have studied details of the molecular origin of slow secondary relaxation near T(g) in a series of neat polyalcohols by means of dielectric spectroscopy and (2)H NMR. From glycerol to threitol, xylitol, and sorbitol the appearance of the secondary
M C Alliegro
Analytical biochemistry, 282(1), 102-106 (2000-06-22)
Dithiothreitol (DTT) is widely used to reduce disulfide bonds in the analysis of protein structure and function. However, thiol-disulfide exchange is not the only mechanism whereby DTT can alter protein function. We observe that DTT diminishes the carbohydrate binding activity
Jonathan D Silk et al.
Journal of immunology (Baltimore, Md. : 1950), 180(10), 6452-6456 (2008-05-06)
Invariant NKT cells (iNKT cells) recognize CD1d/glycolipid complexes. We demonstrate that the nonglycosidic compound threitolceramide efficiently activates iNKT cells, resulting in dendritic cell (DC) maturation and the priming of Ag-specific T and B cells. Threitolceramide-pulsed DCs are more resistant to
F Bravo et al.
Carbohydrate research, 336(2), 83-97 (2001-11-02)
Differently protected erythro and threo furanoid glycals were synthesized by selenoxide elimination when phenyl 1-selenoglycosides were treated in oxidizing conditions (tBuOOH, Ti(O(i)Pr)(4), Et(2)(i)PrN). The phenyl 1-selenoglycosides were obtained from methyl 2-deoxy-D-erythro-pentofuranoside by protection of the primary hydroxyl or both hydroxyls
Justyna Wojno et al.
ACS chemical biology, 7(5), 847-855 (2012-02-14)
Invariant natural killer T (iNKT) cells are restricted by the non-polymorphic MHC class I-like protein, CD1d, and activated following presentation of lipid antigens bound to CD1d molecules. The prototypical iNKT cell agonist is α-galactosyl ceramide (α-GalCer). CD1d-mediated activation of iNKT
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service