All Photos(1)
About This Item
Empirical Formula (Hill Notation):
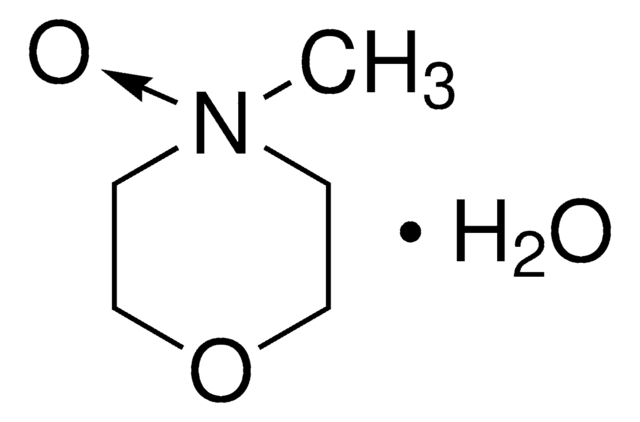
C6H7NO2 · xH2O
CAS Number:
Molecular Weight:
125.13 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
82-85 °C (lit.)
SMILES string
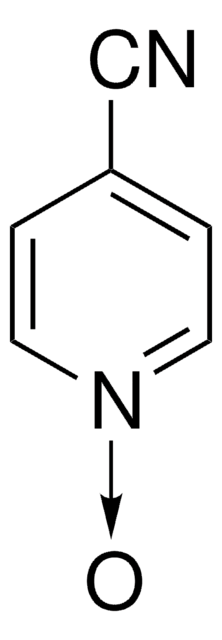
[H]O[H].COc1cc[n+]([O-])cc1
InChI
1S/C6H7NO2.H2O/c1-9-6-2-4-7(8)5-3-6;/h2-5H,1H3;1H2
InChI key
ABJMARLKAXBQBC-UHFFFAOYSA-N
General description
4-Methoxypyridine N-oxide hydrate is a substituted pyridine N-oxide. It forms 1:1 complexes with MnCl2 and NiCl2 salts. Mechanism of reaction between 4-methoxypyridine N-oxide hydrate and bis(pinacolato) (diboron reagent) in CD3CN has been studied by NMR. It forms 1:1 complex with 2,4-dinitrophenol due to the formation of intermolecular hydrogen bond between the O-H and N-O groups.
Application
4-Methoxypyridine N-oxide hydrate may be used in chemical synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hari Prasad Kokatla et al.
The Journal of organic chemistry, 76(19), 7842-7848 (2011-08-05)
Facile reduction of alkylamino-, anilino-, and pyridyl-N-oxides can be achieved via the use of diboron reagents, predominantly bis(pinacolato)- and in some cases bis(catecholato)diboron [(pinB)(2) and (catB)(2), respectively]. Reductions occur upon simply mixing the amine N-oxide and the diboron reagent in
1: 1 Complex of 2, 4-dinitrophenol and 4-methoxypyridine N-oxide hydrate.
Moreno-Fuquen R, et al.
Acta Crystallographica Section E, Structure Reports Online, 57(8), o712-o714 (2001)
Binuclear chlorine-bridged complexes of manganese (II) and nickel (II) chlorides with pyridine N-oxides.
Karayannis NM, et al.
Inorganic Chemistry, 8(12), 2559-2562 (1969)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service