237647
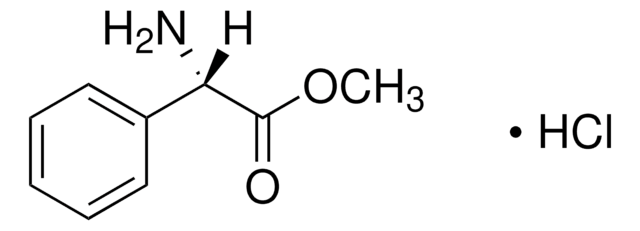
L−(+)-α-Phenylglycine
99%
Synonym(s):
(S)-(+)-2-Phenylglycine, S-(+)-α-Aminophenylacetic acid, L-2-Phenylglycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
151.16
Beilstein:
2208675
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical activity
[α]20/D +155°, c = 1 in 1 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
N[C@H](C(O)=O)c1ccccc1
InChI
1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m0/s1
InChI key
ZGUNAGUHMKGQNY-ZETCQYMHSA-N
Related Categories
Application
Chiral starting material.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shohei Tashiro et al.
Inorganic chemistry, 50(1), 4-6 (2010-12-01)
The optically active cobalt(III) complex with chiral cyclen, (2S,5S,8S,11S)-2,5,8,11-tetraethyl-1,4,7,10-tetraazacyclododecane, preferentially binds to D-phenylglycine (D-Phg) or D-t-leucine (D-t-Leu) rather than L-Phg or L-t-Leu, respectively, with 20% de in dimethyl sulfoxide at 293 K. Comparative studies on the crystal structures of cobalt(III)
Kuoxi Xu et al.
Chirality, 24(8), 646-651 (2012-05-24)
The triazine-based bisbinaphthyl crown ethers oxacalix[2]arene[2]bisbinaphthes R-1, R-2, R-3 and S-1, S-2, S-3 were synthesized. The interactions of these compounds with various α-aminocarboxylic acid anions were studied. The crown ethers were found to carry out highly enantioselective fluorescent recognition of
Hyung Min Kim et al.
The Journal of chemical physics, 128(18), 184313-184313 (2008-06-06)
We investigated the conformational structures of L-phenylglycine in the gas phase by photoionization and double resonance spectroscopy techniques as well as high-level ab initio calculations. The UV-UV and IR-UV double resonance spectroscopy suggested that there exists only one conformer that
Jian-Lian Chen et al.
Analytica chimica acta, 718, 130-137 (2012-02-07)
Three different approaches for immobilizing cross-linked chitosan molecules (CS-s) in sol-gel phases to form chiral OT-CEC capillaries were comparatively investigated in this study. To synthesize column I, a bare capillary was first silanized with triethoxysilane (TEOS) and then reacted with
Motohiro Akazome et al.
The Journal of organic chemistry, 75(3), 660-665 (2010-01-07)
In terms of chiral recognition for racemic aryl methyl sulfoxides in the solid state, three kinds of crystalline (S)-alkylglycyl-(S)-phenylglycines were examined as potential dipeptides host molecules. When (S)-alanyl-(S)-phenylglycines [(S,S)-Ala-Phg] crystallized with aryl methyl sulfoxides, the stereochemistry of preferentially included sulfoxides
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service