163244
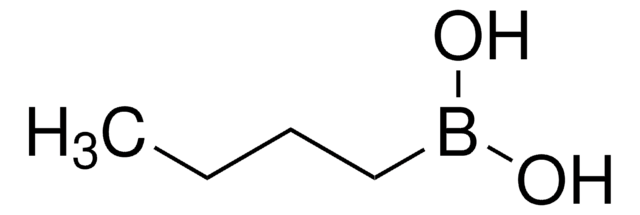
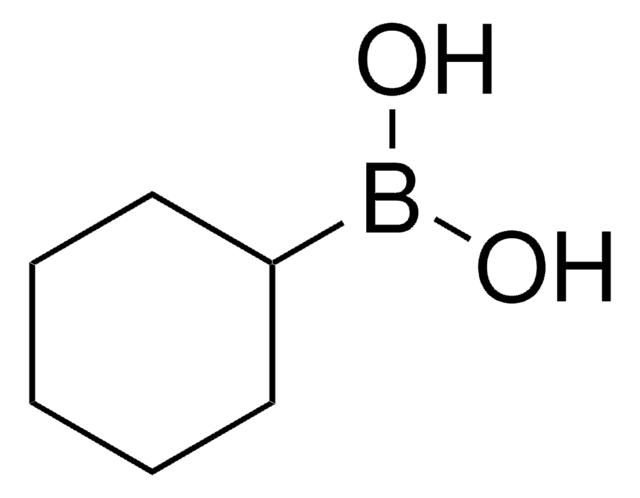
Butylboronic acid
97%
Synonym(s):
1-Butaneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3(CH2)3B(OH)2
CAS Number:
Molecular Weight:
101.94
Beilstein:
1733489
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
90-92 °C (lit.)
SMILES string
CCCCB(O)O
InChI
1S/C4H11BO2/c1-2-3-4-5(6)7/h6-7H,2-4H2,1H3
InChI key
QPKFVRWIISEVCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
A precursor to unsymmetric borinic acids, inhibitors of serine proteases. Reagent used to prepare chiral oxazaborolidines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the American Chemical Society, 116, 3151-3151 (1994)
Tetrahedron Letters, 35, 4419-4419 (1994)
Bioorganic & Medicinal Chemistry Letters, 2, 1391-1391 (1992)
J O Baker et al.
Biochemical and biophysical research communications, 130(3), 1154-1160 (1985-08-15)
The transition-state-analog inhibitor, 1-butaneboronic acid, markedly enhances the uptake of one g-atom of Zn2+ ions from a metal ion buffer system by Zn-depleted Aeromonas aminopeptidase. In contrast, a substrate-analog inhibitor, n-valeramide, does not perturb the equilibrium between Zn2+ ions and
F Ramos et al.
Journal of chromatography. B, Biomedical sciences and applications, 716(1-2), 366-370 (1998-11-21)
A derivatization procedure for confirmatory residue analysis of beta2-agonists is described. Methyl (MBA) and butyl (BBA) boronic acids are simultaneously used for the derivatization of tulobuterol, mabuterol, mapenterol, salbutamol, clenproperol, clenbuterol, clenpenterol and bromobuterol by GC-MS determination. A temperature of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service