141712
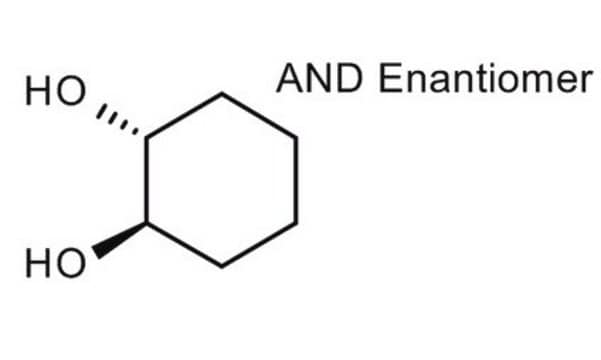
trans-1,2-Cyclohexanediol
98%
Synonym(s):
1,2-trans -Cyclohexanediol, 1,2-trans -Dihydroxycyclohexane, trans -2-Hydroxycyclohexanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
3193810
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
101-104 °C (lit.)
SMILES string
O[C@@H]1CCCC[C@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6-/m1/s1
InChI key
PFURGBBHAOXLIO-PHDIDXHHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C B Shinisha et al.
Organic letters, 11(15), 3242-3245 (2009-09-02)
The relative energies of cyclohexane-1,2-diols and chiral tetrapeptide (2 (Boc) or 3 (Moc)) complexes calculated using DFT indicate a thermodynamic preference for chiral recognition toward (1R,2R)(e,e)-alpha isomer. The barrier for stereoselective acyl transfer is identified as lower for trans-(1R,2R)-cyclohexane-1,2-diol, leading
R J Swift et al.
Applied microbiology and biotechnology, 55(6), 721-726 (2001-08-30)
Benzene dioxygenase (BDO; EC 1.14.12.3) from Pseudomonas putida ML2 dihydroxylates benzene to produce cis-1,2-dihydroxy-cyclohexa-3,5-diene. As well as oxidising benzene and toluene, cell-free extracts of Escherichia coli JM109 expressing recombinant BDO oxidised cyclohexene, 1-methylcyclohexene and 3-methylcyclohexene. In an attempt to construct
Andreas Hartung et al.
The Journal of organic chemistry, 72(26), 10235-10238 (2007-11-16)
Comparison is made between the preparation of trans-1,2-cyclohexanediol in standard glassware (conventional batch production) and in a microreactor (continuous flow production). The reaction sequence involved two exothermic steps where the standard procedure demands slow reagent addition and careful temperature control.
Sigthor Petursson
Carbohydrate research, 338(9), 963-968 (2003-04-12)
The paper reports the tin(II) chloride catalyzed reactions of diazodiphenylmethane with the cis- and trans-1,2-cyclohexanediols and R,S-1,2-propanediol in 1,2-dimethoxyethane and the identification of the monodiphenylmethyl ethers formed. The catalyst is shown to work for both the cis- and trans-cyclohexanediols, but
Yoshihito Shiota et al.
Inorganic chemistry, 50(13), 6200-6209 (2011-06-04)
The catalytic conversion of 1,2-cyclohexanediol to adipic anhydride by Ru(IV)O(tpa) (tpa ═ tris(2-pyridylmethyl)amine) is discussed using density functional theory calculations. The whole reaction is divided into three steps: (1) formation of α-hydroxy cyclohexanone by dehydrogenation of cyclohexanediol, (2) formation of
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service