870432P
Avanti
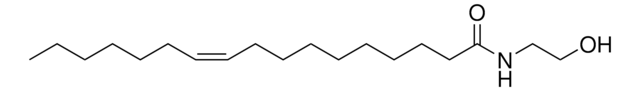
C18:1 anandamide
Avanti Research™ - A Croda Brand 870432P, powder
Synonym(s):
9Z-octadecenoylethanolamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H39NO2
CAS Number:
Molecular Weight:
325.53
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (870432P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 870432P
lipid type
bioactive lipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
O=C(CCCCCCC/C=C\CCCCCCCC)NCCO
General description
Anandamide is an endocannabinoid. It acts as a ligand for cannabinoid (CB) receptors CB1 and CB2 in the brain and peripheral tissues. It is synthesized from glycerophospho-N-arachidonoylethanolamine (GP-NArE) precursor by the reaction catalyzed by glycerophosphodiesterase 1.
Biochem/physiol Actions
Anandamide plays a vital role in various processes including inflammation, pain, and appetite.
Packaging
5 mL Clear Glass Sealed Ampule (870432P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mohammed K Hankir et al.
Frontiers in neuroscience, 10, 620-620 (2017-01-31)
Brain μ-opioid receptors (MORs) stimulate high-fat (HF) feeding and have been implicated in the distinct long term outcomes on body weight of bariatric surgery and dieting. Whether alterations in fat appetite specifically following these disparate weight loss interventions relate to
Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects
Long J, et al.
Nature Chemical Biology, 5(1), 37-37 (2009)
Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain
Astarita G, et al.
Journal of Lipid Research, 49(1), 48-57 (2008)
Mohammad Younus et al.
Langmuir : the ACS journal of surfaces and colloids, 32(35), 8942-8950 (2016-08-16)
Oleoylethanolamide (OEA) is an endogenous lipid with neuroprotective properties and the fortification of its concentration in the brain can be beneficial in the treatment of many neurodegenerative disorders. However, OEA is rapidly eliminated by hydrolysis in vivo, limiting its therapeutic
Gabriel M Simon et al.
The Journal of biological chemistry, 283(14), 9341-9349 (2008-01-30)
Anandamide (AEA) is an endogenous ligand of cannabinoid receptors and a well characterized mediator of many physiological processes including inflammation, pain, and appetite. The biosynthetic pathway(s) for anandamide and its N-acyl ethanolamine (NAE) congeners remain enigmatic. Previously, we proposed an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service