All Photos(1)
About This Item
Empirical Formula (Hill Notation):
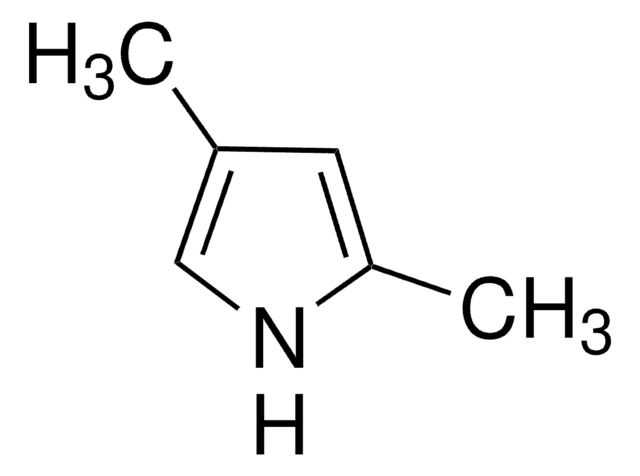
C6H9N
CAS Number:
Molecular Weight:
95.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.505 (lit.)
bp
165 °C/740 mmHg (lit.)
density
0.935 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(C)[nH]1
InChI
1S/C6H9N/c1-5-3-4-6(2)7-5/h3-4,7H,1-2H3
InChI key
PAPNRQCYSFBWDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Ogata et al.
International archives of occupational and environmental health, 62(8), 561-568 (1991-01-01)
High-performance liquid chromatography (HPLC), gas chromatography (GC) and spectrophotometry were used to examine 2,5-dimethylpyrrole, derived from 2,5-hexanedione in the acid-hydrolyzed urine of subjects exposed to n-hexane. The urine of a subject exposed to n-hexane was hydrolyzed with hydrochloric acid and
Determination of urinary 2,5-hexanedione by its conversion to 2,5-dimethylpyrrole.
M Ogata et al.
Industrial health, 28(3), 125-131 (1990-01-01)
Joseph M Beames et al.
The Journal of chemical physics, 131(17), 174305-174305 (2009-11-10)
The photophysical properties of porphyrins have relevance for their use as light-activated drugs in cancer treatment and sensitizers in solid-state solar cells. However, the appearance of their UV-visible spectra is usually explained inadequately by qualitative molecular-orbital theories. We intend to
Xianjie Li et al.
PloS one, 13(12), e0209939-e0209939 (2019-01-01)
Pyrrole adducts are specific reaction products of 2,5-hexadione (2,5-HD) in vivo and are considered highly relevant to the pathogenesis of peripheral nerve impairments after exposure to n-hexane, though the exact mechanism remains unclear. In this study, 40 male Wistar rats
Xianjie Li et al.
Neurotoxicology, 78, 11-20 (2020-02-12)
n-Hexane has been reported to induce serious peripheral neuropathy in workers. Pyrrole adducts are the unique reaction products of n-hexane in organisms and have been demonstrated to be critical to n-hexane neuropathy. Our previous studies have demonstrated that pyrrole adducts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service