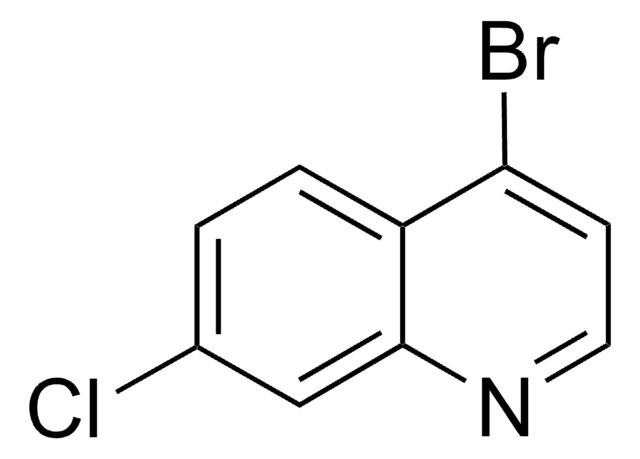
All Photos(1)
About This Item
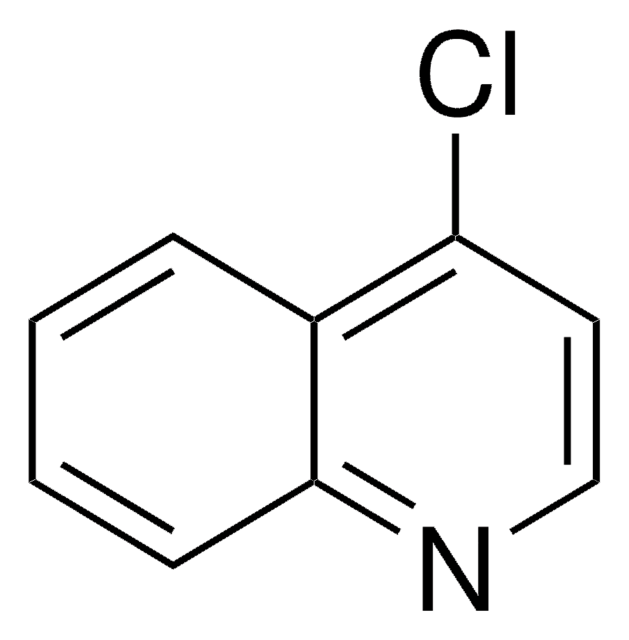
Empirical Formula (Hill Notation):
C9H7ClN2
CAS Number:
Molecular Weight:
178.62
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
form
solid
SMILES string
Nc1ccnc2cc(Cl)ccc12
InChI
1S/C9H7ClN2/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H,(H2,11,12)
InChI key
NDRZSRWMMUGOBP-UHFFFAOYSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Legal Information
Product of BioBlocks
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manolo Casagrande et al.
Bioorganic & medicinal chemistry, 18(18), 6625-6633 (2010-08-28)
A set of nine new arylpyrrolyl derivatives of 7-chloro-4-aminoquinoline, characterized by different substituents on the phenyl ring or different distance between the pyrrolic nitrogen and the 4-aminoquinoline, has been synthesized and tested for their activity against D-10 (CQ-S) and W-2
Igor Opsenica et al.
Journal of medicinal chemistry, 51(19), 6216-6219 (2008-09-09)
The synthesis of the chimeric molecules consisting of two pharmacophores, tetraoxane and 7-chloro-4-aminoquinoline, is reported. The tetraoxanes 2, 4, and 8 show relatively potent in vitro antimalarial activities, with IC90 values for the Plasmodium falciparum strain W2 of 2.26, 12.44
Susanta Roy et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 9(3), 379-383 (2012-09-06)
New derivatives of 7-chloro-4-aminoquinoline Mannich base were prepared by selectively modifying the aliphatic diethyl amino function of isoquine with different aliphatic/aromatic heterocyclic primary amino moieties at Mannich side chain. The synthesized compounds were characterized by their analytical and spectral data
F C Shenton et al.
Transactions of the Royal Society of Tropical Medicine and Hygiene, 82(2), 216-220 (1988-01-01)
Two ELISA tests for detecting chloroquine in urine have been developed using polyclonal and monoclonal antibodies which react with the 7-chloro-4-amino-quinoline part of the chloroquine molecule and thus recognize chloroquine, its metabolites, and amodiaquine. The ELISAs were sensitive and specific
Manolo Casagrande et al.
Bioorganic & medicinal chemistry, 20(19), 5965-5979 (2012-08-25)
With the aim to investigate the effect of different heterocyclic rings linked to the 4-aminoquinoline nucleus on the antimalarial activity, a set of 7-chloro-N-(heteroaryl)-methyl-4-aminoquinoline and 7-chloro-N-(heteroaryl)-4-aminoquinoline was synthesized and tested in vitro against D-10 (CQ-S) and W-2 (CQ-R) strains of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service