78539
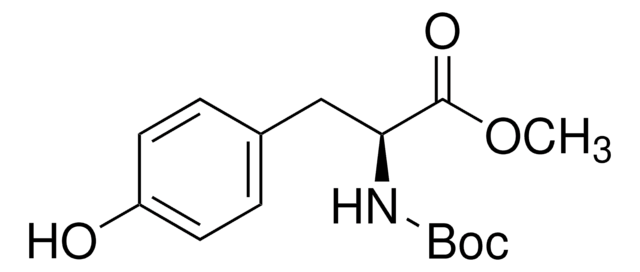
Boc-Tyr(Allyl)-OH
≥98.0% (HPLC)
Synonym(s):
Boc-O-allyl-L-tyrosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H23NO5
CAS Number:
Molecular Weight:
321.37
Beilstein:
3624582
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: Boc solid-phase peptide synthesis
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(=O)N[C@@H](Cc1ccc(OCC=C)cc1)C(O)=O
InChI
1S/C17H23NO5/c1-5-10-22-13-8-6-12(7-9-13)11-14(15(19)20)18-16(21)23-17(2,3)4/h5-9,14H,1,10-11H2,2-4H3,(H,18,21)(H,19,20)/t14-/m0/s1
InChI key
YIRRNENSHUFZBH-AWEZNQCLSA-N
Application
Due to gem-dimethyl substituent effect, Boc-Tyr(Allyl)-OH is found to be resistant to Claisen rearrangement in water.
Other Notes
Allyl group side-chain protected tyrosine; building block for unnatural amino acids by metathesis reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aromatic Claisen Rearrangements of O?Prenylated Tyrosine and Model Prenyl Aryl Ethers: Computational Study of the Role of Water on Acceleration of Claisen Rearrangements.
Osuna S, et al.
European Journal of Organic Chemistry, 2013(14), 2823-2831 (2013)
Angewandte Chemie (International Edition in English), 114, 2964-2964 (2002)
A Loffet et al.
International journal of peptide and protein research, 42(4), 346-351 (1993-10-01)
Allyl and allyloxycarbonyl groups are used for the side-chain protection of amino acids. The protecting groups may be selectively cleaved using the reagent HSnBu3 under palladium catalysis. The preparation of Boc and Fmoc series of protected amino acids is described.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service