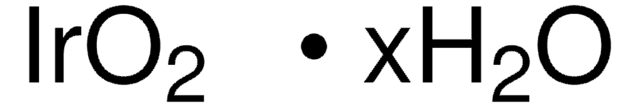463760
Ruthenium(IV) oxide hydrate
99.9% trace metals basis
Synonym(s):
Hydrous ruthenium oxide, Ruthenium dioxide hydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
RuO2 · xH2O
CAS Number:
Molecular Weight:
133.07 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99.9% trace metals basis
form
powder
reaction suitability
core: ruthenium
reagent type: catalyst
SMILES string
O.O=[Ru]=O
InChI
1S/H2O.2O.Ru/h1H2;;;
InChI key
FGEKTVAHFDQHBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aleksandar R Zeradjanin et al.
ChemSusChem, 5(10), 1897-1904 (2012-08-16)
The reaction path of the Cl(2) evolution reaction (CER) was investigated by combining electrochemical and spectroscopic methods. It is shown that oxidation and reconstruction of the catalyst surface during CER is a consequence of the interaction between RuO(2) and water.
O Delmer et al.
Physical chemistry chemical physics : PCCP, 11(30), 6424-6429 (2009-10-08)
The chemical potential of a component of a binary metastable compound is considered in the single-phase and in the compositionally non-variant two-phase regime. A detailed thermodynamic analysis reveals striking differences for identical nominal compositions. Without the loss of generality the
Naiqiang Yan et al.
Environmental science & technology, 45(13), 5725-5730 (2011-06-15)
Catalytic conversion of elemental mercury (Hg(0)) to its oxidized form has been considered as an effective way to enhance mercury removal from coal-fired power plants. In order to make good use of the existing selective catalytic reduction of NO(x) (SCR)
Hiroshi Nishiyama et al.
ChemSusChem, 4(2), 208-215 (2011-02-18)
Sodium, niobium, and tantalum phosphate bronzes Na(4)M(8)P(4)O(32) (M=Nb, Ta) are employed as photocatalysts for water splitting to reveal the effects of the distortion of metal-oxygen octahedra on the photocatalytic performance. Addition of RuO(2) as a co-catalyst leads to high, stable
Li-li Xu et al.
Huan jing ke xue= Huanjing kexue, 28(9), 2009-2013 (2007-11-10)
Electrochemical oxidation ammonia is a new method of ammonia nitrogen wastewater treatment. A study was undertaken of electrochemical oxidation ammonia wastewater in cycle mobil-electrobath. The anode was Ti/RuO2-TiO2-IrO2-SnO2 expanded metal sheet electrode. The cathode was expanded metal sheet electrode. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service