All Photos(1)
About This Item
Linear Formula:
BrC6H4CO2C2H5
CAS Number:
Molecular Weight:
229.07
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.544 (lit.)
bp
131 °C/14 mmHg (lit.)
density
1.403 g/mL at 25 °C (lit.)
functional group
bromo
ester
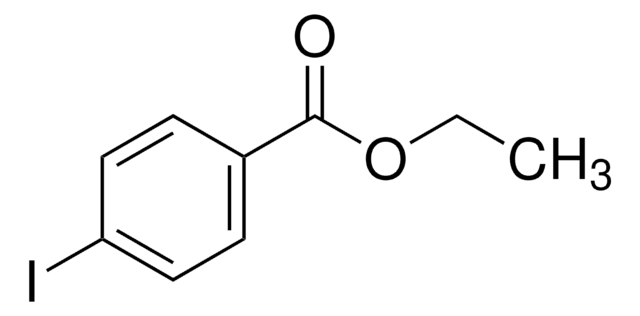
SMILES string
CCOC(=O)c1ccc(Br)cc1
InChI
1S/C9H9BrO2/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6H,2H2,1H3
InChI key
XZIAFENWXIQIKR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 4-bromobenzoate is an ester having electron-withdrawing substituent. It undergoes reduction with potassium diisobutyl-t-butoxyaluminum hydride (PDBBA) at 0°C to yield aldehydes. Reaction of ethyl 4-bromobenzoate and substituted benzyl chloride with zinc dust and a Pd catalyst is reported.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christophe Duplais et al.
Chemical communications (Cambridge, England), 46(4), 562-564 (2010-01-12)
A remarkably simple entry to unsymmetrical diarylmethanes has been developed that relies on an in situ organozinc-mediated, palladium-catalyzed cross-coupling. Thus, by mixing a benzyl and aryl halide together in the presence of Zn metal and a Pd catalyst, diarylmethanes are
Chemoselective Reduction of Esters to Aldehydes by Potassium Diisobutyl-t-butoxyaluminum Hydride (PDBBA).
Chae MJ, et al.
Bull. Korean Chem. Soc., 28(12), 2517-2517 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service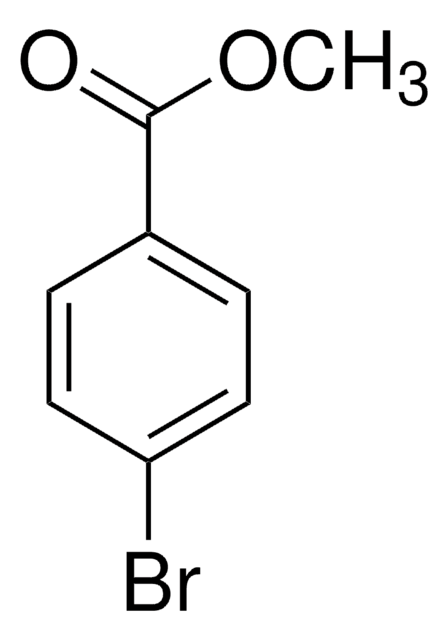






![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



