All Photos(2)
About This Item
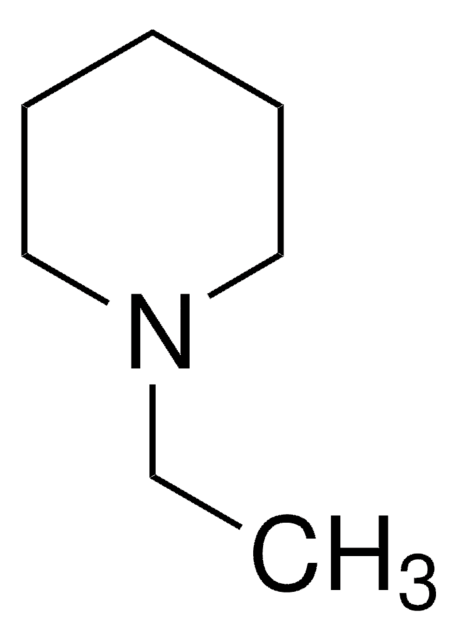
Empirical Formula (Hill Notation):
C8H17N
CAS Number:
Molecular Weight:
127.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.44 (lit.)
bp
155-157 °C/754 mmHg (lit.)
density
0.814 g/mL at 25 °C (lit.)
SMILES string
CCCCN1CCCC1
InChI
1S/C8H17N/c1-2-3-6-9-7-4-5-8-9/h2-8H2,1H3
InChI key
JSHASCFKOSDFHY-UHFFFAOYSA-N
Related Categories
Application
1-Butylpyrrolidine has been used in:
- microwave-assisted synthesis of ionic liquid precursor, 1-butyl-1-methylpyrrolidinium methylcarbonate
- [N,N-methylbutylpyrrolidinium] thiosalicylate, ionic liquid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
96.8 °F - closed cup
Flash Point(C)
36 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Giridhar Pulletikurthi et al.
Dalton transactions (Cambridge, England : 2003), 46(2), 455-464 (2016-12-14)
The mixtures of 1-butylpyrrolidine and ZnCl
Synthesis, characterization and thermal properties of thiosalicylate ionic liquids.
Wilfred CD and Mustafa FB.
Journal of Chemical Sciences (Bangalore), 125(6), 1511-1515 (2013)
Giridhar Pulletikurthi et al.
Chemistry, an Asian journal, 12(20), 2684-2693 (2017-08-05)
Electrostatic interactions are characteristic of ionic liquids (ILs) and play a pivotal role in determining the formation of species when solutes are dissolved in them. The formation of new species/complexes has been investigated for certain ILs. However, such investigations have
Optimised microwave-assisted synthesis of methylcarbonate salts: a convenient methodology to prepare intermediates for ionic liquid libraries.
Holbrey JD, et al.
Green Chemistry, 12(3), 407-413 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service