All Photos(3)
About This Item
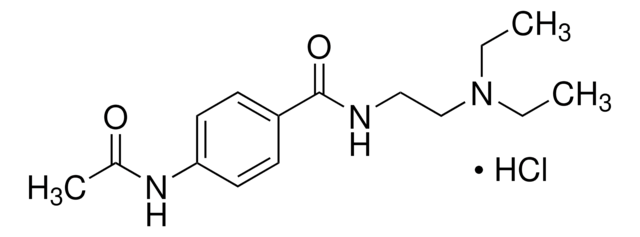
Linear Formula:
4-(CH3CONH)C6H4CONHCH2CH2N(C2H5)2
CAS Number:
Molecular Weight:
277.36
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
solid
mp
138-140 °C (lit.)
solubility
soluble 1%, clear, colorless to faintly yellow (1N HCl)
functional group
amide
amine
SMILES string
CCN(CC)CCNC(=O)c1ccc(NC(C)=O)cc1
InChI
1S/C15H23N3O2/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19)
InChI key
KEECCEWTUVWFCV-UHFFFAOYSA-N
General description
The relaxant effects of N-acetylprocainamide on bovine tracheal smooth muscle was studied.
Application
N-acetylprocainamide (NAPA) was used as a model drug in the study of establishing a quantitative approach to predict the renal clearances of basic drugs using N-1-methylnicotinamide (NMN).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yoo-Seong Jeong et al.
Pharmaceutics, 11(3) (2019-03-09)
Previous observations demonstrated that cimetidine decreased the clearance of procainamide (PA) and/or N-acetylprocainamide (NAPA; the primary metabolite of PA) resulting in the increased systemic exposure and the decrease of urinary excretion. Despite an abundance of in vitro and in vivo
N D Eddington et al.
Biopharmaceutics & drug disposition, 19(5), 291-296 (1998-07-23)
The effect of moderate and prolonged exercise on the disposition and metabolism of drugs has not been extensively examined. The present study examined the effect of exercise training on the pharmacokinetics of procainamide and its active metabolite, N-acetylprocainamide. Male Sprague
J R Koup et al.
Therapeutic drug monitoring, 20(1), 73-77 (1998-03-05)
Procainamide hydrochloride is a Class 1A antiarrhythmic agent administered intravenously or orally for treatment of symptomatic ventricular premature depolarizations (VPD), nonsustained ventricular tachycardia, and life-threatening ventricular arrhythmias. A new sustained-release formulation, Procanbid, which allows for twice-daily dosing was recently approved
F A Brightman et al.
Drug metabolism and disposition: the biological fate of chemicals, 34(1), 94-101 (2005-10-14)
Estimation of xenobiotic kinetics in humans frequently relies upon extrapolation from experimental data generated in animals. In an accompanying paper, we have presented a unique, generic, physiologically based pharmacokinetic model and described its application to the prediction of rat plasma
B B Yang et al.
Journal of clinical pharmacology, 36(7), 623-633 (1996-07-01)
A study was conducted to evaluate the pharmacokinetics of procainamide and its active metabolite, N-acetylprocainamide (NAPA), as a function of dose and formulation and to characterize the relationship between ventricular premature depolarization (VPD) rate and plasma concentrations of procainamide and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service