All Photos(1)
About This Item
Empirical Formula (Hill Notation):
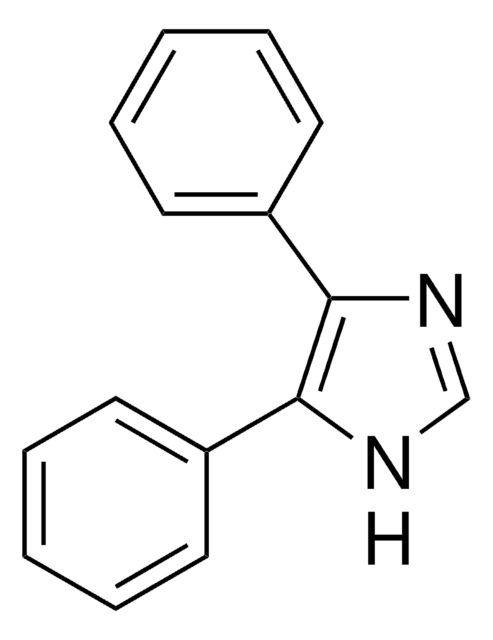
C9H8N2
CAS Number:
Molecular Weight:
144.17
Beilstein:
2969
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
128-131 °C (lit.)
solubility
acetone: soluble 25 mg/mL, clear, colorless to yellow (typical)
functional group
phenyl
SMILES string
c1ccc(cc1)-c2c[nH]cn2
InChI
1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11)
InChI key
XHLKOHSAWQPOFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Phenylimidazole was used to investigate the protein-ligand interactions in cytochrome P450 from the thermoacidophile Picrophilus torridus. It was used as heme ligand during the crystallization of recombinant human indoleamine 2,3-dioxygenase. It was used in the synthesis of complexes of copper and cobalt.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J K Yano et al.
The Journal of biological chemistry, 275(40), 31086-31092 (2000-06-22)
The structure of the first P450 identified in Archaea, CYP119 from Sulfolobus solfataricus, has been solved in two different crystal forms that differ by the ligand (imidazole or 4-phenylimidazole) coordinated to the heme iron. A comparison of the two structures
Winny W Ho et al.
Biochemistry, 47(7), 2071-2079 (2008-01-17)
The crystal structure of a cytochrome P450 from the thermoacidophile Picrophilus torridus, CYP231A2 (PTO1399), has been solved. This structure reveals a wide open substrate access channel. To better understand ligand-induced structural transitions in CYP231A2, protein-ligand interactions were investigated using 4-phenylimidazole.
T Kimura et al.
Journal of medicinal chemistry, 36(11), 1641-1653 (1993-05-28)
In our continuing search to find systemically bioavailable ACAT (acyl-CoA:cholesterol O-acyltransferase) inhibitors with more potent antiatherosclerotic effect than N-[2-(dimethylamino)-6-[3-(5-methyl-4-phenyl-1H-imidazol-1-yl)propoxy] phenyl]-N'-pentylurea (3), a series of phenylureas linked to 4-phenylimidazole were synthesized and evaluated for in vitro inhibitory activity toward both aortic
M Sono et al.
Biochemistry, 28(13), 5392-5399 (1989-06-27)
The effects of norharman, one of the few known inhibitors of the heme protein indoleamine 2,3-dioxygenase, and of 4-phenylimidazole (4-PheImid), a heme ligand, on the catalytic (Vmax, Km) and spectroscopic properties (optical absorption, CD, and magnetic CD) of the rabbit
Y K Li et al.
Journal of biochemistry, 123(3), 416-422 (1998-05-30)
Series of 4-arylimidazoles, omega-N-acylhistamines and 4-(omega-phenylalkyl)imidazoles were synthesized in order to probe the active site topology of sweet almond beta-glucosidase. These imidazole derivatives were shown to be very powerful competitive inhibitors. Among the 20 tested compounds, omega-N-benzoylhistamine and 4-(3'-phenylpropyl)imidazole are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service